In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
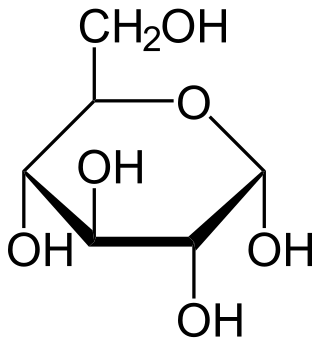
Glucose is a sugar with the molecular formula C6H12O6. Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world.

Formic acid, systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure H−C(=O)−O−H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts and the anion derived from formic acid are called formates. Industrially, formic acid is produced from methanol.

Pectin is a heteropolysaccharide, a structural acid contained in the primary lamella, in the middle lamella, and in the cell walls of terrestrial plants. The principal chemical component of pectin is galacturonic acid which was isolated and described by Henri Braconnot in 1825. Commercially produced pectin is a white-to-light-brown powder, produced from citrus fruits for use as an edible gelling agent, especially in jams and jellies, dessert fillings, medications, and sweets; and as a food stabiliser in fruit juices and milk drinks, and as a source of dietary fiber.

Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway, spearmint, and dill.

N,N′-Dicyclohexylcarbodiimide (DCC or DCCD) is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. Its primary use is to couple amino acids during artificial peptide synthesis. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water.
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH3)2NC5H4N. This white solid is of interest because it is more basic than pyridine, owing to the resonance stabilisation from the NMe2 substituent.

Pentyl is a five-carbon alkyl group or substituent with chemical formula -C5H11. It is the substituent form of the alkane pentane.

1-Propanol is a primary alcohol with the formula CH3CH2CH2OH and sometimes represented as PrOH or n-PrOH. It is a colourless liquid and an isomer of 2-propanol. 1-Propanol is used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters, and, sometimes, as a disinfecting agent.

Isoamyl acetate, also known as isopentyl acetate, is an ester formed from isoamyl alcohol and acetic acid, with the molecular formula . It is a colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents. Isoamyl acetate has a strong odor which is described as similar to both banana and pear. Pure isoamyl acetate, or mixtures of isoamyl acetate, amyl acetate, and other flavors in ethanol may be referred to as banana oil or pear oil.

Isoamyl alcohol is a colorless liquid with the formula C
5H
12O, specifically (H3C–)2CH–CH2–CH2–OH. It is one of several isomers of amyl alcohol (pentanol). It is also known as isopentyl alcohol, isopentanol, or (in the IUPAC recommended nomenclature) 3-methyl-butan-1-ol. An obsolete name for it was isobutyl carbinol.

2-Ethylhexanol (abbreviated 2-EH) is an organic compound with formula C8H18O. It is a branched, eight-carbon chiral alcohol. It is a colorless liquid that is poorly soluble in water but soluble in most organic solvents. It is produced on a large scale (>2,000,000,000 kg/y) for use in numerous applications such as solvents, flavors, and fragrances and especially as a precursor for production of other chemicals such as emollients and plasticizers. It is encountered in plants, fruits, and wines. The odor has been reported as "heavy, earthy, and slightly floral" for the R enantiomer and "a light, sweet floral fragrance" for the S enantiomer.
The Yamaguchi esterification is the chemical reaction of an aliphatic carboxylic acid and 2,4,6-trichlorobenzoyl chloride to form a mixed anhydride which, upon reaction with an alcohol in the presence of stoichiometric amount of DMAP, produces the desired ester. It was first reported by Masaru Yamaguchi et al. in 1979.

Isobutyl formate (2-methylpropyl methanoate) is an organic ester with the chemical formula C5H10O2. It is formed by the Fischer esterification of isobutanol with formic acid, with the aid of an acid catalyst. It is used as a flavor and fragrance ingredient because of its odor which is sweet, ethereal, and slightly fruity.

Cholesterol total synthesis in chemistry describes the total synthesis of the complex biomolecule cholesterol and is considered a great scientific achievement. The research group of Robert Robinson with John Cornforth published their synthesis in 1951 and that of Robert Burns Woodward with Franz Sondheimer in 1952. Both groups competed for the first publication since 1950 with Robinson having started in 1932 and Woodward in 1949. According to historian Greg Mulheirn the Robinson effort was hampered by his micromanagement style of leadership and the Woodward effort was greatly facilitated by his good relationships with chemical industry. Around 1949 steroids like cortisone were produced from natural resources but expensive. Chemical companies Merck & Co. and Monsanto saw commercial opportunities for steroid synthesis and not only funded Woodward but also provided him with large quantities of certain chemical intermediates from pilot plants. Hard work also helped the Woodward effort: one of the intermediate compounds was named Christmasterone as it was synthesized on Christmas Day 1950 by Sondheimer.
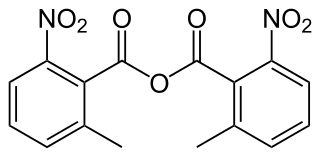
2-Methyl-6-nitrobenzoic anhydride is an organic acid anhydride also known as the Shiina reagent, having a structure wherein carboxylic acids undergo intermolecular dehydration condensation. It was developed in 2002 by Prof. Isamu Shiina. The compound is often abbreviated MNBA.
Shiina macrolactonization is an organic chemical reaction that synthesizes cyclic compounds by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic cyclization method using Lewis acid catalyst, and, in 2002, a basic cyclization using nucleophilic catalyst.
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. A colorless liquid at room temperature, it has the semi-developed formula of CH3(CH2)6COOCH2CH3, and is used in food industries as a flavoring and in the perfume industry as a scent additive. It is present in many fruits and alcoholic beverages, and has a strong odor of fruit and flowers. It is used in the creation of synthetic fruity scents.
Shiina esterification is an organic chemical reaction that synthesizes carboxylic esters from nearly equal amounts of carboxylic acids and alcohols by using aromatic carboxylic acid anhydrides as dehydration condensation agents. In 1994, Prof. Isamu Shiina reported an acidic coupling method using Lewis acid, and, in 2002, a basic esterification using nucleophilic catalyst.