Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).
Enoyl-CoA-(∆) isomerase (EC 5.3.3.8, also known as dodecenoyl-CoA- isomerase, 3,2-trans-enoyl-CoA isomerase, ∆3 ,∆2 -enoyl-CoA isomerase, or acetylene-allene isomerase, is an enzyme that catalyzes the conversion of cis- or trans-double bonds of coenzyme A bound fatty acids at gamma-carbon to trans double bonds at beta-carbon as below:
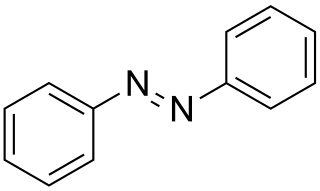
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes.
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria:

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.
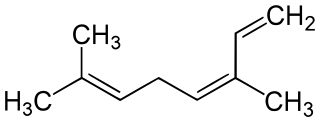
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is cis-3,7-dimethyl-1,3,7-octatriene. β-Ocimene is trans-3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, cis and trans, with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent, and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air. Like other terpenes, the ocimenes are nearly insoluble in water, but soluble in common organic solvents.
In inorganic chemistry, the trans effect is the increased lability of ligands that are trans to certain other ligands, which can thus be regarded as trans-directing ligands. It is attributed to electronic effects and it is most notable in square planar complexes, although it can also be observed for octahedral complexes. The analogous cis effect is most often observed in octahedral transition metal complexes.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.

Platinum(II) chloride is the chemical compound PtCl2. It is an important precursor used in the preparation of other platinum compounds. It exists in two crystalline forms, but the main properties are somewhat similar: dark brown, insoluble in water, diamagnetic, and odorless.

Cyclododecatrienes are cyclic trienes with the formula C12H18. Four isomers are known for 1,5,9-cyclododecatriene. The trans,trans,cis-isomer is a precursor in the production of nylon-12.
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines.

Chrysanthemic acid is an organic compound that is related to a variety of natural and synthetic insecticides. It is related to the pyrethrin I and II, as well as the pyrethroids. One of the four stereoisomers, (1R,3R)- or (+)-trans-chrysanthemic acid (pictured), is the acid part of the ester pyrethrin I, which occurs naturally in the seed cases of Chrysanthemum cinerariaefolium. Many synthetic pyrethroids, for example the allethrins, are esters of all four stereoisomers. Staudinger and Ružička named chrysanthemic acid in 1924.
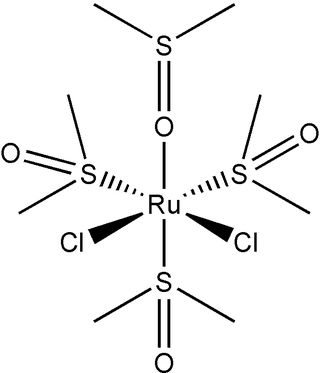
Dichlorotetrakis(dimethyl sulfoxide) ruthenium(II) describes coordination compounds with the formula RuCl2(dmso)4, where DMSO is dimethylsulfoxide. Both cis and trans isomers are known, but the cis isomer is more common. The cis isomer is a yellow, air-stable solid that is soluble in some organic solvents. These compounds have attracted attention as possible anti-cancer drugs.
In chemistry, hyponitrite may refer to the anion N
2O2−
2 ([ON=NO]2−), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R1−O−N=N−O−R2, where R1 and R2 are organic groups. Such compounds can be viewed as salts and esters of respectively hyponitrous acid H
2N
2O
2 or HON=NOH.
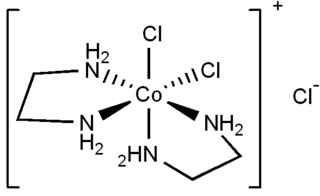
cis-Dichlorobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCl2(en)2]Cl (en = ethylenediamine). The salt consists of a cationic coordination complex and a chloride anion. It is a violet diamagnetic solid that is soluble in water. One chloride ion in this salt readily undergoes ion exchange, but the two other chlorides are less reactive, being bound to the metal center.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.
In organic chemistry, enone–alkene cycloadditions are a version of the [2+2] cycloaddition This reaction involves an enone and alkene as substrates. Although the concerted photochemical [2+2] cycloaddition is allowed, the reaction between enones and alkenes is stepwise and involves discrete diradical intermediates.

In organic chemistry, the Mallory reaction is a photochemical-cyclization–elimination reaction of diaryl-ethylene structures to form phenanthrenes and other polycyclic form polycyclic aromatic hydrocarbons and heteroaromatics. This name reaction is named for Frank Mallory, who discovered it while a graduate student.

Sodium hyponitrite is a solid ionic compound with formula Na
2N
2O
2 or (Na+
)2[ON=NO]2−.













