Plant hormones are signal molecules, produced within plants, that occur in extremely low concentrations. Plant hormones control all aspects of plant growth and development, including embryogenesis, the regulation of organ size, pathogen defense, stress tolerance and reproductive development. Unlike in animals each plant cell is capable of producing hormones. Went and Thimann coined the term "phytohormone" and used it in the title of their 1937 book.

Jasmonate (JA) and its derivatives are lipid-based plant hormones that regulate a wide range of processes in plants, ranging from growth and photosynthesis to reproductive development. In particular, JAs are critical for plant defense against herbivory and plant responses to poor environmental conditions and other kinds of abiotic and biotic challenges. Some JAs can also be released as volatile organic compounds (VOCs) to permit communication between plants in anticipation of mutual dangers.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids, and alkaloids; however, the term applies to any phytochemicals that are induced by microbial infection.

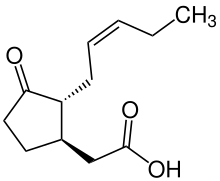
Methyl jasmonate is a volatile organic compound used in plant defense and many diverse developmental pathways such as seed germination, root growth, flowering, fruit ripening, and senescence. Methyl jasmonate is derived from jasmonic acid and the reaction is catalyzed by S-adenosyl-L-methionine:jasmonic acid carboxyl methyltransferase.

The innate immune system or nonspecific immune system is one of the two main immunity strategies in vertebrates. The innate immune system is an alternate defense strategy and is the dominant immune system response found in plants, fungi, prokaryotes, and invertebrates.
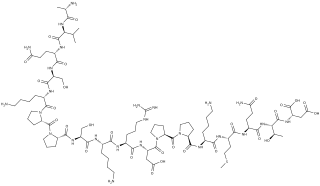
Systemin is a plant peptide hormone involved in the wound response in the family Solanaceae. It was the first plant hormone that was proven to be a peptide having been isolated from tomato leaves in 1991 by a group led by Clarence A. Ryan. Since then, other peptides with similar functions have been identified in tomato and outside of the Solanaceae. Hydroxyproline-rich glycopeptides were found in tobacco in 2001 and AtPeps were found in Arabidopsis thaliana in 2006. Their precursors are found both in the cytoplasm and cell walls of plant cells, upon insect damage, the precursors are processed to produce one or more mature peptides. The receptor for systemin was first thought to be the same as the brassinolide receptor but this is now uncertain. The signal transduction processes that occur after the peptides bind are similar to the cytokine-mediated inflammatory immune response in animals. Early experiments showed that systemin travelled around the plant after insects had damaged the plant, activating systemic acquired resistance, now it is thought that it increases the production of jasmonic acid causing the same result. The main function of systemins is to coordinate defensive responses against insect herbivores but they also affect plant development. Systemin induces the production of protease inhibitors which protect against insect herbivores, other peptides activate defensins and modify root growth. They have also been shown to affect plants' responses to salt stress and UV radiation. AtPEPs have been shown to affect resistance against oomycetes and may allow A. thaliana to distinguish between different pathogens. In Nicotiana attenuata, some of the peptides have stopped being involved in defensive roles and instead affect flower morphology.
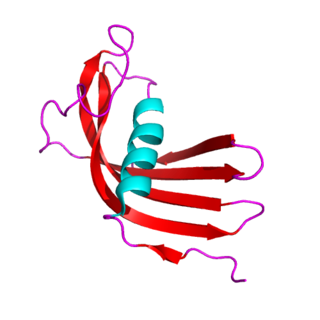
The cystatins are a family of cysteine protease inhibitors which share a sequence homology and a common tertiary structure of an alpha helix lying on top of an anti-parallel beta sheet. The family is subdivided as described below.

The octadecanoid pathway is a biosynthetic pathway for the production of the phytohormone jasmonic acid (JA), an important hormone for induction of defense genes. JA is synthesized from alpha-linolenic acid, which can be released from the plasma membrane by certain lipase enzymes. For example, in the wound defense response, phospholipase C will cause the release of alpha-linolenic acid for JA synthesis.

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.

In enzymology, an allene-oxide cyclase is an enzyme that belongs to the family of isomerases, specifically a class of other intramolecular oxidoreductases. The systematic name of this enzyme class is (9Z)-(13S)-12,13-epoxyoctadeca-9,11,15-trienoate isomerase (cyclizing).
Biotic stress is stress that occurs as a result of damage done to an organism by other living organisms, such as bacteria, viruses, fungi, parasites, beneficial and harmful insects, weeds, and cultivated or native plants. It is different from abiotic stress, which is the negative impact of non-living factors on the organisms such as temperature, sunlight, wind, salinity, flooding and drought. The types of biotic stresses imposed on an organism depend the climate where it lives as well as the species' ability to resist particular stresses. Biotic stress remains a broadly defined term and those who study it face many challenges, such as the greater difficulty in controlling biotic stresses in an experimental context compared to abiotic stress.

A loline alkaloid is a member of the 1-aminopyrrolizidines, which are bioactive natural products with several distinct biological and chemical features. The lolines are insecticidal and insect-deterrent compounds that are produced in grasses infected by endophytic fungal symbionts of the genus Epichloë. Lolines increase resistance of endophyte-infected grasses to insect herbivores, and may also protect the infected plants from environmental stresses such as drought and spatial competition. They are alkaloids, organic compounds containing basic nitrogen atoms. The basic chemical structure of the lolines comprises a saturated pyrrolizidine ring, a primary amine at the C-1 carbon, and an internal ether bridge—a hallmark feature of the lolines, which is uncommon in organic compounds—joining two distant ring carbons. Different substituents at the C-1 amine, such as methyl, formyl, and acetyl groups, yield loline species that have variable bioactivity against insects. Besides endophyte–grass symbionts, loline alkaloids have also been identified in some other plant species; namely, Adenocarpus species and Argyreia mollis.
In plant biology, elicitors are extrinsic or foreign molecules often associated with plant pests, diseases or synergistic organisms. Elicitor molecules can attach to special receptor proteins located on plant cell membranes. These receptors are able to recognize the molecular pattern of elicitors and trigger intracellular defence signalling via the octadecanoid pathway. This response results in the enhanced synthesis of metabolites which reduce damage and increase resistance to pest, disease or environmental stress. This is an immune response called pattern triggered immunity (PTI).
Bacterial blight of cotton is a disease affecting the cotton plant resulting from infection by Xanthomonas axonopodis pathovar malvacearum (Xcm) a Gram negative, motile rod-shaped, non spore-forming bacterium with a single polar flagellum

Tritrophic interactions in plant defense against herbivory describe the ecological impacts of three trophic levels on each other: the plant, the herbivore, and its natural enemies. They may also be called multitrophic interactions when further trophic levels, such as soil microbes, endophytes, or hyperparasitoids are considered. Tritrophic interactions join pollination and seed dispersal as vital biological functions which plants perform via cooperation with animals.
Induced systemic resistance (ISR) is a resistance mechanism in plants that is activated by infection. Its mode of action does not depend on direct killing or inhibition of the invading pathogen, but rather on increasing physical or chemical barrier of the host plant. Like the Systemic Acquired Resistance (SAR) a plant can develop defenses against an invader such as a pathogen or parasite if an infection takes place. In contrast to SAR, which is triggered by the accumulation of salicylic acid, ISR instead relies on signal transduction pathways activated by jasmonate and ethylene.

In organic chemistry, an allene oxide is an epoxide of an allene. The parent allene oxide is CH2=C(O)CH2 (CAS RN 40079-14-9), a rare and reactive species of only theoretical interest. Typical allene oxides require steric protection for their isolation. Certain derivatives can be prepared by epoxidation of the allenes with peracetic acid. Allene oxides tend to rearrange to cyclopropanones.
In plant biology, proteinase inhibitors are a family of small proteins that serve an integral role in the plant’s defense mechanisms against herbivory from insects or microorganisms that may compromise the integrity of the plant.
Plants are constantly exposed to different stresses that result in wounding. Plants have adapted to defend themselves against wounding events, like herbivore attacks or environmental stresses. There are many defense mechanisms that plants rely on to help fight off pathogens and subsequent infections. Wounding responses can be local, like the deposition of callose, and others are systemic, which involve a variety of hormones like jasmonic acid and abscisic acid.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.