
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Unlike most antibiotics, antiviral drugs do not destroy their target pathogen; instead they inhibit its development.

Pectin is a structural acidic heteropolysaccharide contained in the primary and middle lamella and cell walls of terrestrial plants. Its main component is galacturonic acid, a sugar acid derived from galactose. It was first isolated and described in 1825 by Henri Braconnot. It is produced commercially as a white to light brown powder, mainly extracted from citrus fruits, and is used in food as a gelling agent, particularly in jams and jellies. It is also used in dessert fillings, medicines, sweets, as a stabiliser in fruit juices and milk drinks, and as a source of dietary fiber.

Citrus depressa (Citrus × depressa, formerly C. pectinifera, Okinawan: シークヮーサー/シークァーサー, romanized: shiikwaasa, Japanese: ヒラミレモン, romanized: hirami remon or シークワーサー, shiikuwāsā, in English sometimes called shiikuwasha, shequasar, Taiwan tangerine, Okinawa lime, flat lemon, hirami lemon, or thin-skinned flat lemon, is a small, green citrus fruit rich in flavonoids and native to East Asia.
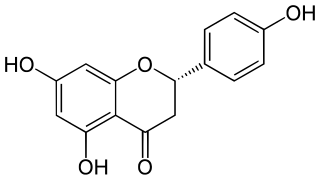
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs.

Naringin is a flavanone-7-O-glycoside between the flavanone naringenin and the disaccharide neohesperidose. The flavonoid naringin occurs naturally in citrus fruits, especially in grapefruit, where naringin is responsible for the fruit's bitter taste. In commercial grapefruit juice production, the enzyme naringinase can be used to remove the bitterness created by naringin. In humans naringin is metabolized to the aglycone naringenin by naringinase present in the gut.
An aglycone is the compound remaining after the glycosyl group on a glycoside is replaced by a hydrogen atom. For example, the aglycone of a cardiac glycoside would be a steroid molecule.
Grapefruit seed extract (GSE), also known as citrus seed extract, is a liquid extract derived from the seeds, pulp, and white membranes of grapefruit. GSE is prepared by grinding the grapefruit seed and juiceless pulp, then mixing with glycerin. Commercially available GSEs sold to consumers are made from the seed, pulp, and glycerin blended together. GSE is sold as a dietary supplement and is used in cosmetics.
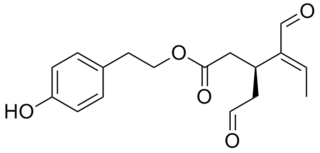
Oleocanthal is a phenylethanoid, or a type of natural phenolic compound found in extra-virgin olive oil. It appears to be responsible for the burning sensation that occurs in the back of the throat when consuming such oil. Oleocanthal is a tyrosol ester and its chemical structure is related to oleuropein, also found in olive oil.
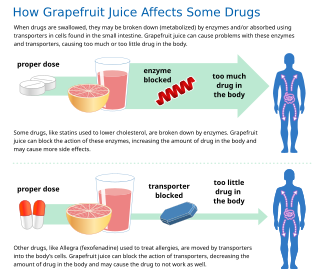
Some fruit juices and fruits can interact with numerous drugs, in many cases causing adverse effects. The effect was first discovered accidentally, when a test of drug interactions with alcohol used grapefruit juice to hide the taste of the ethanol.

Helenalin, or (-)-4-Hydroxy-4a,8-dimethyl-3,3a,4a,7a,8,9,9a-octahydroazuleno[6,5-b]furan-2,5-dione, is a toxic sesquiterpene lactone which can be found in several plants such as Arnica montana and Arnica chamissonis Helenalin is responsible for the toxicity of the Arnica spp. Although toxic, helenalin possesses some in vitro anti-inflammatory and anti-neoplastic effects. Helenalin can inhibit certain enzymes, such as 5-lipoxygenase and leukotriene C4 synthase. For this reason the compound or its derivatives may have potential medical applications.

Osteonectin (ON) also known as secreted protein acidic and rich in cysteine (SPARC) or basement-membrane protein 40 (BM-40) is a protein that in humans is encoded by the SPARC gene.

Ursolic acid, is a pentacyclic triterpenoid identified in the epicuticular waxes of apples as early as 1920 and widely found in the peels of fruits, as well as in herbs and spices like rosemary and thyme.

Honokiol is a lignan isolated from the bark, seed cones, and leaves of trees belonging to the genus Magnolia. It has been identified as one of the chemical compounds in some traditional eastern herbal medicines along with magnolol, 4-O-methylhonokiol, and obovatol.

Galangin is a flavonol, a type of flavonoid.
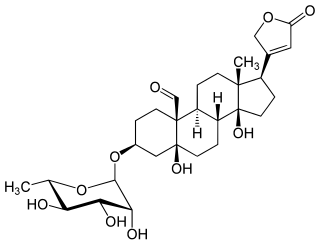
Convallatoxin is a glycoside extracted from Convallaria majalis.

Withaferin A is a steroidal lactone, derived from Acnistus arborescens, Withania somnifera and other members of family Solanaceae. It has been traditionally used in ayurvedic medicine. It is the first member of the withanolide class of ergostane type product to be discovered. This natural product has wide range of pharmacological activities including cardioprotective, anti-inflammatory, immuno-modulatory, anti-angiogenesis, anti-metastasis and anti-carcinogenic properties.

3-Deazaneplanocin A is a drug which acts as both a S-adenosylhomocysteine synthesis inhibitor and also a histone methyltransferase EZH2 inhibitor. Studies have shown that it has in vitro against a variety of different tumor cell lines.
Cynaropicrin is a sesquiterpene lactone of the guaianolide type found mainly in leaves of artichoke plants. It is one of the compounds that gives the artichoke its characteristic bitterness. It is found in artichoke leaves with an abundance of approximately 87 g/kg, but can hardly be found in other parts of the plant. Cynaropicrin makes up about 0.7% of leaf extracts of the artichoke. It exhibits a large diversity of bioactivities and shows properties such as anti-inflammatory, antifeedant and activation of bitter sensory receptors, but has not yet been used in medicine. Despite its pharmacologically beneficial properties, it can be toxic in higher doses. The compound has attracted attention in recent years as a potential anticancer drug.

Lipase inhibitors belong to a drug class that is used as an antiobesity agent. Their mode of action is to inhibit gastric and pancreatic lipases, enzymes that play an important role in the digestion of dietary fat. Lipase inhibitors are classified in the ATC-classification system as A08AB . Numerous compounds have been either isolated from nature, semi-synthesized, or fully synthesized and then screened for their lipase inhibitory activity but the only lipase inhibitor on the market is orlistat . Lipase inhibitors have also shown anticancer activity, by inhibiting fatty acid synthase.

Lobucavir is an antiviral drug that shows broad-spectrum activity against herpesviruses, hepatitis B and other hepadnaviruses, HIV/AIDS and cytomegalovirus. It initially demonstrated positive results in human clinical trials against hepatitis B with minimal adverse effects but was discontinued from further development following the discovery of increased risk of cancer associated with long-term use in mice. Although this carcinogenic risk is present in other antiviral drugs, such as zidovudine and ganciclovir that have been approved for clinical use, development was halted by Bristol-Myers Squibb, its manufacturer.


















