
Soap is a salt of a fatty acid used in a variety of cleansing and lubricating products. In a domestic setting, soaps are surfactants usually used for washing, bathing, and other types of housekeeping. In industrial settings, soaps are used as thickeners, components of some lubricants, and precursors to catalysts.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances which react with acids as originally proposed by G.-F. Rouelle in the mid-18th century.
Saponification is a process that involves the conversion of fat, oil, or lipid, into soap and alcohol by the action of aqueous alkali. Soaps are salts of fatty acids, which in turn are carboxylic acids with long carbon chains. A typical soap is sodium oleate.

Stearic acid ( STEER-ik, stee-ARR-ik) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a waxy solid and its chemical formula is C17H35CO2H. Its name comes from the Greek word στέαρ "stéar", which means tallow. The salts and esters of stearic acid are called stearates. As its ester, stearic acid is one of the most common saturated fatty acids found in nature following palmitic acid. The triglyceride derived from three molecules of stearic acid is called stearin.
The Monsanto process is an industrial method for the manufacture of acetic acid by catalytic carbonylation of methanol. The Monsanto process has largely been supplanted by the Cativa process, a similar iridium-based process developed by BP Chemicals Ltd which is more economical and environmentally friendly.
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.
Grease is a solid or semisolid lubricant formed as a dispersion of thickening agents in a liquid lubricant. Grease generally consists of a soap emulsified with mineral or vegetable oil.

Magnesium stearate is the chemical compound with the formula Mg(C
18H
35O
2)
2. It is a soap, consisting of salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate is a white, water-insoluble powder. Its applications exploit its softness, insolubility in many solvents, and low toxicity. It is used as a release agent and as a component or lubricant in the production of pharmaceuticals and cosmetics.
Sodium stearate is the sodium salt of stearic acid. This white solid is the most common soap. It is found in many types of solid deodorants, rubbers, latex paints, and inks. It is also a component of some food additives and food flavorings.
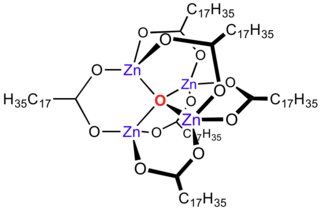
Zinc stearate is a "zinc soap" that is widely used industrially. In this context, soap is used in its formal sense, a metal salt of a fatty acid: in this case stearic acid. It is a white solid that repels water. It is insoluble in polar solvents such as alcohol and ether but soluble in aromatic hydrocarbons and chlorinated hydrocarbons when heated. It is the most powerful mold release agent among all metal soaps. It contains no electrolyte and has a hydrophobic effect. Its main application areas are the plastics and rubber industry, where it is used as a releasing agent and lubricant which can be easily incorporated.

Lithium soap is a soap consisting of a lithium salt of a fatty acid. Sodium-based and potassium-based soaps are used as cleaning agents in domestic and industrial applications, whereas lithium soaps are used as components of lithium grease.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si 3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.

Calcium stearate is a carboxylate salt of calcium, classified as a calcium soap. The salt is a component of some lubricants, surfactants, as well as many foodstuffs. It is a white waxy powder.
Soap scum or lime soap is the white solid composed of calcium stearate, magnesium stearate, and similar alkaline earth metal derivatives of fatty acids. These materials result from the addition of soap and other anionic surfactants to hard water. Hard water contains calcium and magnesium ions, which react with the surfactant anion to give these metallic or lime soaps.
Lithium 12-hydroxystearate (C18H35LiO3) is a chemical compound classified as a lithium soap. In chemistry, "soap" refers to salts of fatty acids. Lithium 12-hydroxystearate is a white solid. Lithium soaps are key component of many lubricating greases.
Cadmium stearate is a salt with the formula Cd(O2CC17H35)2. Classified as a metallic soap, this a white solid is used as a lubricant and as a heat- and light-stabilizer in polyvinyl chloride. It is produced by the reaction of cadmium chloride with sodium stearate or heating stearic acid and cadmium oxide or hydroxide. The use of cadmium stearate is being phased out because of its toxicity.
A metallic soap is a metallic salt of a fatty acid. Theoretically, soaps can be made of any metal, although not all enjoy practical uses. Varying the metal can strongly affect the properties of the compound, particularly its solubility.

Lithium cyanide is an inorganic compound with the chemical formula LiCN. It is a toxic, white colored, hygroscopic, water-soluble salt that finds only niche uses.

2-Octanol is an organic compound with the chemical formula CH3CH(OH)(CH2)5CH3. It is a colorless oily liquid that is poorly soluble in water but soluble in most organic solvents. 2-Octanol is classified fatty alcohol. A secondary alcohol, it is chiral, although it is mainly encountered as a racemic mixture.

Lithium lactate is a chemical compound, a salt of lithium and lactic acid with the formula CH3CH(OH)COOLi, an amorphous solid, very soluble in water.










