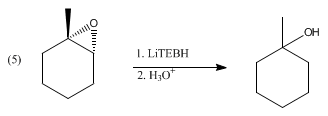| |
| Names | |
|---|---|
| Preferred IUPAC name Lithium triethylboranuide | |
| Other names Superhydride LiTEBH | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.040.963 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Li(C2H5)3BH | |
| Molar mass | 105.95 g/mol |
| Appearance | Colorless to yellow liquid |
| Density | 0.890 g/cm3, liquid |
| Boiling point | 66 °C (151 °F; 339 K) for THF |
| reactive | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards | highly flammable corrosive Causes burns Probable Carcinogen |
| GHS labelling: [1] | |
   | |
| Danger | |
| H250, H260, H314, H335 | |
| P210, P222, P223, P231+P232, P260, P261, P264, P271, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P335+P334, P363, P370+P378, P402+P404, P403+P233, P405, P422, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related hydride | Lithium borohydride sodium borohydride sodium hydride lithium aluminium hydride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
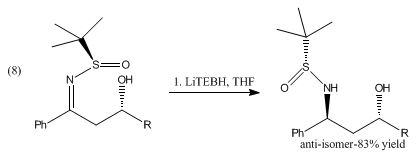
Lithium triethylborohydride is the organoboron compound with the formula Li Et3 BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. [2] The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.
Contents
LiBHEt3 is a stronger reducing agent than lithium borohydride and lithium aluminium hydride.