
Moths are a group of insects that includes all members of the order Lepidoptera that are not butterflies. They were previously classified as suborder Heterocera, but the group is paraphyletic with respect to butterflies and neither subordinate taxa are used in modern classifications. Moths make up the vast majority of the order. There are thought to be approximately 160,000 species of moth, many of which have yet to be described. Most species of moth are nocturnal, although there are also crepuscular and diurnal species.

Lymantria dispar, also known as the gypsy moth or the spongy moth, is an Eurasian species of moth in the family Erebidae. Lymantria dispar is subdivided into several subspecies, with subspecies such as L. d. dispar and L. d. japonica being clearly identifiable without ambiguity. Lymantria dispar has been introduced to several continents and is now found in Europe, Africa, Asia, North America and South America. The polyphagous larvae live on a variety of deciduous and coniferous trees and can cause severe damage in years of mass reproduction. Due to these features, Lymantria dispar is listed among the world's 100 worst invasive alien species.

The Lymantriinae are a subfamily of moths of the family Erebidae. The taxon was erected by George Hampson in 1893.

Baculoviridae is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera.

The cabbage looper is a medium-sized moth in the family Noctuidae, a family commonly referred to as owlet moths. Its common name comes from its preferred host plants and distinctive crawling behavior. Cruciferous vegetables, such as cabbage, bok choy, and broccoli, are its main host plant; hence, the reference to cabbage in its common name. The larva is called a looper because it arches its back into a loop when it crawls.

Orgyia leucostigma, the white-marked tussock moth, is a moth in the family Erebidae. The species was first described by James Edward Smith in 1797. The caterpillar is very common especially in late summer in eastern North America, extending as far west as Texas, California, and Alberta.

Calosoma sycophanta, the forest caterpillar hunter, is a species of ground beetle belonging to the family Carabidae.

Hyblaea puera, the teak defoliator, is a moth and cryptic species complex native to South Asia and South-east Asia. It was first described by Pieter Cramer in 1777. The species has also been recently reported to be present in Central America and Africa. The caterpillar feeds on teak and other trees. It is considered to be one of the major teak pests around the world.
Animal viruses are viruses that infect animals. Viruses infect all cellular life and although viruses infect every animal, plant, fungus and protist species, each has its own specific range of viruses that often infect only that species.

Glyptapanteles is a genus of endoparasitoid wasps found in all continents, except Antarctica. The larvae of the members of Glyptapanteles sp. are distinguished by their ability to manipulate their hosts into serving as bodyguards.

Malacosoma californicum, the western tent caterpillar, is a moth of the family Lasiocampidae. It is a tent caterpillar. The Western Tent Caterpillar is found in southern Canada, the western United States, and parts of northern Mexico. There are currently six recognized subspecies of M. californicum. Western tent caterpillars are gregarious and will spend a large portion of their time with other caterpillars in silken tents constructed during their larval stage.

Lasiocampa quercus, the oak eggar, is a common moth of the family Lasiocampidae found in Europe, including Britain and Ireland. It feeds on a variety of plant species, and may develop over two years in higher latitudes, where it may be known as the northern eggar. Its specific name quercus refers to the fact that its cocoon generally resembles an acorn, not that its primary food source is oak.

The gypsy moth, also known as the spongy moth, was introduced in 1868 into the United States by Étienne Léopold Trouvelot, a French scientist living in Medford, Massachusetts. Because native silk-spinning caterpillars were susceptible to disease, Trouvelot imported the species in order to breed a more resistant hybrid species. Some of the moths escaped, found suitable habitat, and began breeding. The gypsy moth is now a major pest of hardwood trees in the Eastern United States.

Compsilura concinnata is a parasitoid native to Europe that was introduced to North America in 1906 to control the population of an exotic forest, univoltine, spongy moth named Lymantria dispar. It is an endoparasitoid of larvae and lives with its host for most of its life. Eventually the parasitoid ends up killing the host and occasionally eating it. It attacks over 200 host species, mainly insects from the Orders: Coleoptera, Lepidoptera and Hymenoptera. Since this parasite has the ability to attack many different types of hosts, the organism has spilled over from the intended forest systems into other areas, like agricultural fields, affecting cabbage pests including the cabbage looper (Trichoplusia); the cabbage worm ; and even other invasive species such as the brown-tail moth. However, it also attacks native, non-pest insects such as the Cecropia moth and American moon moth.

Lymantria dispar asiatica, the LDA moth or Asian spongy moth, also known as the Asian gypsy moth, is a moth in the family Erebidae of Eurasian origin. It is similar to Lymantria dispar dispar in appearance, but adult females can fly. It is classified as a pest and is host to over 500 species of trees, shrubs and plants.

Lymantria dispar dispar or LDD moth, commonly known as the gypsy moth, European gypsy moth, North American gypsy moth, or spongy moth, is a species of moth in the family Erebidae that is of Eurasian origin. It has a range that extends over Europe, Africa, and North America.

Lymantria dispar japonica, also known as the Japanese gypsy moth, is a moth in the family Erebidae of Eurasian origin.

Entomophaga maimaiga is a Japanese fungus which has shown striking success in managing spongy moth populations in North America.
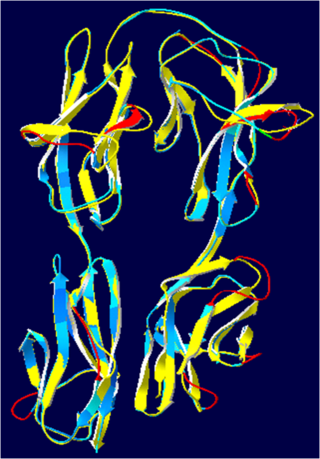
Hemolin is an immunoglobulin-like protein exclusively found in Lepidoptera. It was first discovered in immune-challenged pupae of Hyalophora cecropia and Manduca sexta.
Dinocampus coccinellae paralysis virus (DcPV) is a single-stranded, positive-sense RNA virus of insects, in the picorna-like virus family Iflaviridae, which was first characterised in 2015. It asymptomatically infects the parasitic braconid wasp, Dinocampus coccinellae, and has been proposed to be associated with the paralytic effect the wasp has on its host, the spotted lady beetle, Coleomegilla maculata, which it turns into a so-called "zombie bodyguard" for its pupa.

















