Related Research Articles
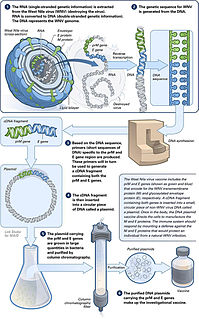
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence onto the cells of an immunized species.
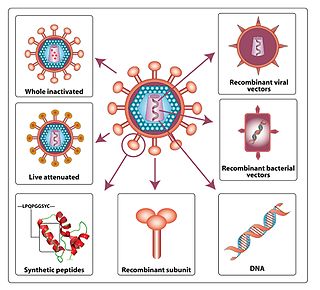
An HIV vaccine could be either a preventive vaccine or a therapeutic vaccine, which means it will either protect individuals from being infected with HIV or treat HIV-infected individuals. It could either induce an immune response against HIV or consist of preformed antibodies against HIV.

Vaccinia virus is a large, complex, enveloped virus belonging to the poxvirus family. It has a linear, double-stranded DNA genome approximately 190 kbp in length, which encodes approximately 250 genes. The dimensions of the virion are roughly 360 × 270 × 250 nm, with a mass of approximately 5–10 fg. The vaccinia virus is the source of the modern smallpox vaccine, which the World Health Organisation used to eradicate smallpox in a global vaccination campaign in 1958–1977. Although smallpox no longer exists in the wild, vaccinia virus is still studied widely by scientists as a tool for gene therapy and genetic engineering.
MVA85A is a vaccine against tuberculosis developed by researchers led by Professor Helen McShane at Oxford University. This vaccine produces higher levels of long-lasting cellular immunity when used together with the older TB vaccine BCG. Phase I clinical trials were completed and then phase II clinical trials took place in South Africa. Efficacy trials ran in parallel from 2009 to 2019.
This is a list of AIDS-related topics, many of which were originally taken from the public domain U.S. Department of Health Glossary of HIV/AIDS-Related Terms, 4th Edition.
The Modified Vaccinia Ankara (MVA) is an attenuated vaccine of a poxvirus. It was licensed and used as a poxvirus vaccine in Bavaria and is a vector for vaccination against non-poxvirus diseases.
GeoVax is a clinical-stage biotechnology company initially established primarily to develop an effective and safe vaccine against many serious human diseases for which there are significant unmet medical needs. GeoVax’ novel development platform builds on the proven clinical and commercial success of the Modified Vaccinia Ankara (MVA) vector technology, with improvements to antigen design and manufacturing capabilities. GeoVax technology approach uses recombinant DNA or recombinant viruses to produce virus-like particles (VLPs) in the person being vaccinated. In human clinical trials of the company’s HIV vaccines, GeoVax demonstrated that VLPs are safe and eliciting both strong and durable humoral and cellular immune response.
The United States Military HIV Research Program was initiated by the United States Congress in 1986, in reaction to the threat of lost effectiveness of U.S./Allied troops due to HIV infection. The mission of MHRP is to develop an HIV-1 vaccine, provide prevention, care, and treatment, and conduct meaningful HIV/AIDS research for the global community through the President's Emergency Plan for AIDS Relief (PEPFAR). It is centered at the Walter Reed Army Institute of Research (WRAIR), and has established five international research sites in Africa and Asia. MHRP also partners with the Armed Forces Research Institute of Medical Sciences (AFRIMS) in Thailand. MHRP works closely with The Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), most notably in the development of the RV144 HIV vaccine in Thailand. MHRP is the largest research program supported by the HJF.
Enzo Paoletti was an Italian-American virologist who developed the technology to express foreign antigens in vaccinia and other poxviruses. This advance led to the development of vaccines against multiple disease-causing pathogens.
The STEP Study was a Phase IIb clinical trial intended to study the efficacy of an experimental HIV vaccine based on a human adenovirus 5 (HAdV-5) vector. The study was conducted in North and South America, the Caribbean, and Australia. A related study using the same experimental vaccine was conducted simultaneously in South Africa. These trials were co-sponsored by Merck, the HIV Vaccine Trials Network (HVTN), and the National Institute of Allergy and Infectious Diseases (NIAID), and had an Oversight Committee consisting of representatives from these three organizations. In South Africa the trial was overseen by the South African AIDS Vaccine Initiative.
JX-594 is an oncolytic virus is designed to target and destroy cancer cells. It is also known as Pexa-Vec, INN pexastimogene devacirepvec) and was constructed in Dr. Edmund Lattime's lab at Thomas Jefferson University, tested in clinical trials on melanoma patients, and licensed and further developed by SillaJen.
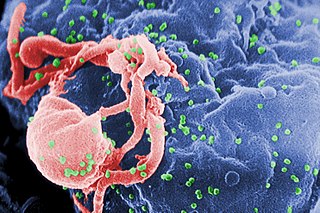
HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Transgene SA is a France-based fully integrated biopharmaceutical company specialising in immunotherapeutics to treat cancer and infectious diseases. The company has subsidiaries in China and in the USA. The company has been strongly supported by the Mérieux family since 1994. Transgene was listed on the Paris Stock Exchange in 1998.

Bavarian Nordic A/S is a fully integrated biotechnology company focused on the development, manufacturing and commercialization of cancer immunotherapies and vaccines for infectious diseases. The company is headquartered in Kvistgaard, Denmark, where it also operates a commercial-scale manufacturing facility. The company has a research and development facility in Martinsried, Germany and an office in Morrisville, North Carolina.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.
Anna-Lise WilliamsonMASSAf is a Professor of Virology at the University of Cape Town. Williamson obtained her PhD from the University of the Witwatersrand in 1985. Her area of expertise is human papillomavirus, but is also known on an international level for her work in developing vaccines for HIV. These vaccines have been introduce in phase 1 of clinical trial. Williamson has published more than 120 papers.
Julianna Lisziewicz is a Hungarian immunologist. Lisziewicz headed many research teams that have discovered and produced immunotheraputic drugs to treat diseases like cancer and chronic infections like HIV/AIDS. Some of these drugs have been successfully used in clinical trials.
B13R is a protein expressed by vaccinia virus.
ChAdOx1 is an adenoviral vector vaccine developed by the Jenner Institute, University of Oxford. The vector is a chimpanzee adenovirus modified to avoid its replication.
A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material coding for a desired antigen into the recipient's host cells. As of April 2021, six viral vector vaccines have been authorized in at least one country: four COVID-19 vaccines and two Ebola vaccines.
References
- 1 2 3 4 5 Jesus Diaz (September 28, 2011). "This 90 Percent Successful Vaccine May Be Our Best Chance to Eradicate AIDS". Gizmodo . Retrieved September 29, 2011.
- ↑ Stephen Adams (September 29, 2011). "HIV could be 'minor infection' with new vaccine". Irish Independent . Retrieved September 29, 2011.
- 1 2 Gopalan T (September 28, 2011). "Promising Show By HIV Vaccine MVA-B In Clinical Trials". Medindia . Retrieved September 29, 2011.
- ↑ Ylva Mossing (September 29, 2011). "Vaccin gör HIV till "mindre infektion"". Aftonbladet (in Swedish). Retrieved September 29, 2011.
- ↑ Mothe B, et al. (February 26, 2015). "Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVA-B) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1". Journal of Antimicrobial Chemotherapy . 70 (6): 1833–1842. doi: 10.1093/jac/dkv046 . Retrieved June 25, 2021.
- ↑ Guardo AC, et al. (October 24, 2017). "Safety and vaccine-induced HIV-1 immune responses in healthy volunteers following a late MVA-B boost 4 years after the last immunization". PLOS One . doi:10.1371/journal.pone.0186602. PMC 5655491 . Retrieved June 25, 2021.
- ↑ Perez P, et al. (February 6, 2020). "Deletion of Vaccinia Virus A40R Gene Improves the Immunogenicity of the HIV-1 Vaccine Candidate MVA-B". Vaccines . 8 (1): 70. doi:10.3390/vaccines8010070. PMC 7158668 . Retrieved June 25, 2021.