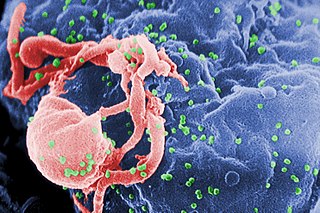
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.

Feline immunodeficiency virus (FIV) is a Lentivirus that affects cats worldwide, with 2.5% to 4.4% of felines being infected.

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.
Following infection with HIV-1, the rate of clinical disease progression varies between individuals. Factors such as host susceptibility, genetics and immune function, health care and co-infections as well as viral genetic variability may affect the rate of progression to the point of needing to take medication in order not to develop AIDS.
The genome and proteins of HIV have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.

DC-SIGN also known as CD209 is a protein which in humans is encoded by the CD209 gene.
HIV-associated neurocognitive disorders (HAND) are neurological disorders associated with HIV infection and AIDS. It is a syndrome of progressive deterioration of memory, cognition, behavior, and motor function in HIV-infected individuals during the late stages of the disease, when immunodeficiency is severe. HAND may include neurological disorders of various severity. HIV-associated neurocognitive disorders are associated with a metabolic encephalopathy induced by HIV infection and fueled by immune activation of macrophages and microglia. These cells are actively infected with HIV and secrete neurotoxins of both host and viral origin. The essential features of HIV-associated dementia (HAD) are disabling cognitive impairment accompanied by motor dysfunction, speech problems and behavioral change. Cognitive impairment is characterised by mental slowness, trouble with memory and poor concentration. Motor symptoms include a loss of fine motor control leading to clumsiness, poor balance and tremors. Behavioral changes may include apathy, lethargy and diminished emotional responses and spontaneity. Histopathologically, it is identified by the infiltration of monocytes and macrophages into the central nervous system (CNS), gliosis, pallor of myelin sheaths, abnormalities of dendritic processes and neuronal loss.

Lck is a 56 kDa protein that is found inside specialized cells of the immune system called lymphocytes. The Lck is a member of Src kinase family (SFK), it is important for the activation of the T-cell receptor signaling in both naive T cells and effector T cells. The role of the Lck is less prominent in the activation or in the maintenance of memory CD8 T cells in comparison to CD4 T cells. In addition, the role of the lck varies among the memory T cells subsets. It seems that in mice, in the effector memory T cells (TEM) population, more than 50% of lck is present in a constitutively active conformation, whereas, only less than 20% of lck is present as active form of lck. These differences are due to differential regulation by SH2 domain–containing phosphatase-1 (Shp-1) and C-terminal Src kinase.

CD28 is one of the proteins expressed on T cells that provide co-stimulatory signals required for T cell activation and survival. T cell stimulation through CD28 in addition to the T-cell receptor (TCR) can provide a potent signal for the production of various interleukins.
Jurkat cells are an immortalized line of human T lymphocyte cells that are used to study acute T cell leukemia, T cell signaling, and the expression of various chemokine receptors susceptible to viral entry, particularly HIV. Jurkat cells can produce interleukin 2, and are used in research involving the susceptibility of cancers to drugs and radiation.
Visna-maedi virus from the genus Lentivirus and subfamily Orthoretrovirinae, is a retrovirus that causes encephalitis and chronic pneumonitis in sheep. It is known as visna when found in the brain, and maedi when infecting the lungs. Lifelong, persistent infections in sheep occur in the lungs, lymph nodes, spleen, joints, central nervous system, and mammary glands; The condition is sometimes known as ovine progressive pneumonia (OPP), particularly in the United States, or Montana sheep disease. White blood cells of the monocyte/macrophage lineage are the main target of the virus.

T-cell surface glycoprotein CD3 zeta chain also known as T-cell receptor T3 zeta chain or CD247 is a protein that in humans is encoded by the CD247 gene.

T-cell surface glycoprotein CD3 gamma chain is a protein that in humans is encoded by the CD3G gene.

Phosphatidylinositol 3-kinase catalytic subunit type 3 is an enzyme subunit that in humans is encoded by the PIK3C3 gene. It's a class III phosphoinositide 3-kinase.

Nuclear factor of activated T-cells, cytoplasmic 4 is a protein that in humans is encoded by the NFATC4 gene.
Christopher Edward Rudd, is a Canadian-born immunologist-biochemist. He is currently Professor of Medicine at the Universite de Montreal and Director, Immunology-Oncology at the Centre de Recherche Hôpital Maisonneuve-Rosemont (CR-HMR).

In molecular biology, Tat is a protein that is encoded for by the tat gene in HIV-1. Tat is a regulatory protein that drastically enhances the efficiency of viral transcription. Tat stands for "Trans-Activator of Transcription". The protein consists of between 86 and 101 amino acids depending on the subtype. Tat vastly increases the level of transcription of the HIV dsDNA. Before Tat is present, a small number of RNA transcripts will be made, which allow the Tat protein to be produced. Tat then binds to cellular factors and mediates their phosphorylation, resulting in increased transcription of all HIV genes, providing a positive feedback cycle. This in turn allows HIV to have an explosive response once a threshold amount of Tat is produced, a useful tool for defeating the body's response.

Vpr is a Human immunodeficiency virus gene and protein product.
Bovine immunodeficiency virus (BIV) is a retrovirus belonging to the genus Lentivirus. It is similar to the human immunodeficiency virus (HIV) and infects cattle. The cells primarily infected are lymphocytes and monocytes/macrophages.
Vpx is a virion-associated protein encoded by human immunodeficiency virus type 2 HIV-2 and most simian immunodeficiency virus (SIV) strains, but that is absent from HIV-1. It is similar in structure to the protein Vpr that is carried by SIV and HIV-2 as well as HIV-1. Vpx is one of five accessory proteins carried by lentiviruses that enhances viral replication by inhibiting host antiviral factors.












