Related Research Articles
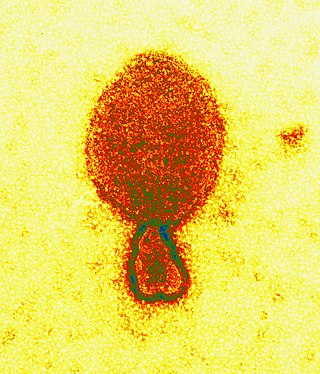
Henipavirus is a genus of negative-strand RNA viruses in the family Paramyxoviridae, order Mononegavirales containing six established species, and numerous others still under study. Henipaviruses are naturally harboured by several species of small mammals, notably pteropid fruit bats, microbats of several species, and shrews. Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.

Respiratory syncytial virus (RSV), also called human respiratory syncytial virus (hRSV) and human orthopneumovirus, is a contagious virus that causes infections of the respiratory tract. It is a negative-sense, single-stranded RNA virus. Its name is derived from the large cells known as syncytia that form when infected cells fuse.

Human metapneumovirus is a negative-sense single-stranded RNA virus of the family Pneumoviridae and is closely related to the Avian metapneumovirus (AMPV) subgroup C. It was isolated for the first time in 2001 in the Netherlands by using the RAP-PCR technique for identification of unknown viruses growing in cultured cells. As of 2016, it was the second most common cause of acute respiratory tract illness in otherwise-healthy children under the age of 5 in a large US outpatient clinic.

Measles morbillivirus(MeV), also called measles virus (MV), is a single-stranded, negative-sense, enveloped, non-segmented RNA virus of the genus Morbillivirus within the family Paramyxoviridae. It is the cause of measles. Humans are the natural hosts of the virus; no animal reservoirs are known to exist.

Gregory Antone Prince is an American pathology researcher, businessman, author, social critic, and historian of the Latter Day Saint movement.

Influenza C virus is the only species in the genus Gammainfluenzavirus, in the virus family Orthomyxoviridae, which like other influenza viruses, causes influenza.

Jaagsiekte sheep retrovirus (JSRV) is a betaretrovirus which is the causative agent of a contagious lung cancer in sheep, called ovine pulmonary adenocarcinoma.
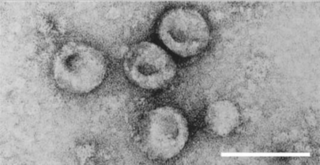
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
Rous sarcoma virus (RSV) is a retrovirus and is the first oncovirus to have been described. It causes sarcoma in chickens.

Murine respirovirus, formerly Sendai virus (SeV) and previously also known as murine parainfluenza virus type 1 or hemagglutinating virus of Japan (HVJ), is an enveloped, 150-200 nm–diameter, negative sense, single-stranded RNA virus of the family Paramyxoviridae. It typically infects rodents and it is not pathogenic for humans or domestic animals.
Palivizumab, sold under the brand name Synagis, is a monoclonal antibody produced by recombinant DNA technology used to prevent severe disease caused by respiratory syncytial virus (RSV) infections. It is recommended for infants at high-risk for RSV due to conditions such as prematurity or other medical problems including heart or lung diseases.

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. The suboptimal antibodies can result from natural infection or from vaccination. ADE may cause enhanced respiratory disease, but is not limited to respiratory disease. It has been observed in HIV, RSV virus and Dengue virus and is monitored for in vaccine development.
2F5 is a broadly neutralizing human monoclonal antibody (mAb) that has been shown to bind to and neutralize HIV-1 in vitro, making it a potential candidate for use in vaccine synthesis. 2F5 recognizes an epitope in the membrane-proximal external region (MPER) of HIV-1 gp41. 2F5 then binds to this epitope and its constant region interacts with the viral lipid membrane, which neutralizes the virus.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.

Human coronavirus HKU1 (HCoV-HKU1) is a species of coronavirus in humans and animals. It causes an upper respiratory disease with symptoms of the common cold, but can advance to pneumonia and bronchiolitis. It was first discovered in January 2004 from one man in Hong Kong. Subsequent research revealed it has global distribution and earlier genesis.

Pneumoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Humans, cattle, and rodents serve as natural hosts. Respiratory tract infections are associated with member viruses such as human respiratory syncytial virus. There are five species in the family which are divided between the genera Metapneumovirus and Orthopneumovirus. The family used to be considered as a sub-family of Paramyxoviridae, but has been reclassified as of 2016.
A respiratory syncytial virus vaccine, or RSV vaccine, is a vaccine that protects against respiratory syncytial virus. RSV affects an estimated 64 million people and causes 160,000 deaths worldwide each year.
Jason S. McLellan is a structural biologist, professor in the Department of Molecular Biosciences and Robert A. Welch Chair in Chemistry at The University of Texas at Austin who specializes in understanding the structure and function of viral proteins, including those of coronaviruses. His research focuses on applying structural information to the rational design of vaccines and other therapies for viruses, including SARS-CoV-2, the novel coronavirus that causes COVID-19, and respiratory syncytial virus (RSV). McLellan and his team collaborated with researchers at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center to design a stabilized version of the SARS-CoV-2 spike protein, which biotechnology company Moderna used as the basis for the vaccine mRNA-1273, the first COVID-19 vaccine candidate to enter phase I clinical trials in the U.S. At least three other vaccines use this modified spike protein: those from Pfizer and BioNTech; Johnson & Johnson and Janssen Pharmaceuticals; and Novavax.

The nucleocapsid (N) protein is a protein that packages the positive-sense RNA genome of coronaviruses to form ribonucleoprotein structures enclosed within the viral capsid. The N protein is the most highly expressed of the four major coronavirus structural proteins. In addition to its interactions with RNA, N forms protein-protein interactions with the coronavirus membrane protein (M) during the process of viral assembly. N also has additional functions in manipulating the cell cycle of the host cell. The N protein is highly immunogenic and antibodies to N are found in patients recovered from SARS and COVID-19.
William Paul Duprex is a British scientist and advocate for vaccines and global health. He serves as Director of the University of Pittsburgh's Center for Vaccine Research and Regional Biocontainment Laboratory. Duprex holds the Jonas Salk Chair in Vaccine Research. He is also a professor of microbiology and molecular genetics at the University of Pittsburgh School of Medicine and serves as Editor-in-Chief of the Journal of General Virology, which is published by the Microbiology Society, and a senior editor of mSphere, published by the American Society for Microbiology. Duprex is an expert in measles and mumps viruses and studies viral spillover from animals to humans, including the SARS-CoV-2 virus that caused the COVID-19 pandemic. Duprex is a Fellow of the American Academy of Microbiology.
References
- ↑ Harcourt J, Alvarez R, Jones LP, Henderson C, Anderson LJ, Tripp RA (February 2006). "Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses". Journal of Immunology. 176 (3): 1600–8. doi: 10.4049/jimmunol.176.3.1600 . PMID 16424189.
- ↑ Li XQ, Fu ZF, Alvarez R, Henderson C, Tripp RA (January 2006). "Respiratory syncytial virus (RSV) infects neuronal cells and processes that innervate the lung by a process involving RSV G protein". Journal of Virology. 80 (1): 537–40. doi:10.1128/JVI.80.1.537-540.2006. PMC 1317531 . PMID 16352577.
- ↑ Zlateva KT, Lemey P, Moës E, Vandamme AM, Van Ranst M (July 2005). "Genetic variability and molecular evolution of the human respiratory syncytial virus subgroup B attachment G protein". Journal of Virology. 79 (14): 9157–67. doi:10.1128/JVI.79.14.9157-9167.2005. PMC 1168771 . PMID 15994810.
- ↑ Li X, Sambhara S, Li CX, Ettorre L, Switzer I, Cates G, James O, Parrington M, Oomen R, Du RP, Klein M (March 2000). "Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate". Virology. 269 (1): 54–65. doi: 10.1006/viro.2000.0186 . PMID 10725198.
- ↑ Anderson LJ, Jadhao SJ, Paden CR, Tong S (July 2021). "Functional Features of the Respiratory Syncytial Virus G Protein". Viruses. 13 (7): 1214. doi: 10.3390/v13071214 . PMC 8310105 . PMID 34372490.