Related Research Articles
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis, they release new infectious virus particles or virions to help destroy the remaining tumour. Oncolytic viruses are thought not only to cause direct destruction of the tumour cells, but also to stimulate host anti-tumour immune system responses.
Viral encephalitis is inflammation of the brain parenchyma, called encephalitis, by a virus. The different forms of viral encephalitis are called viral encephalitides. It is the most common type of encephalitis and often occurs with viral meningitis. Encephalitic viruses first cause infection and replicate outside of the central nervous system (CNS), most reaching the CNS through the circulatory system and a minority from nerve endings toward the CNS. Once in the brain, the virus and the host's inflammatory response disrupt neural function, leading to illness and complications, many of which frequently are neurological in nature, such as impaired motor skills and altered behavior.

Virus latency is the ability of a pathogenic virus to lie dormant (latent) within a cell, denoted as the lysogenic part of the viral life cycle. A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny without the host becoming reinfected by new outside virus, and stays within the host indefinitely.
HHV Latency Associated Transcript is a length of RNA which accumulates in cells hosting long-term, or latent, Human Herpes Virus (HHV) infections. The LAT RNA is produced by genetic transcription from a certain region of the viral DNA. LAT regulates the viral genome and interferes with the normal activities of the infected host cell.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word herpein, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. Currently, 107 species are recognized, all but one of which are in one of the three subfamilies.

Human Herpes Virus (HHV) Infected Cell Polypeptide 0 (ICP0) is a protein, encoded by the DNA of herpes viruses. It is produced by herpes viruses during the earliest stage of infection, when the virus has recently entered the host cell; this stage is known as the immediate-early or α ("alpha") phase of viral gene expression. During these early stages of infection, ICP0 protein is synthesized and transported to the nucleus of the infected host cell. Here, ICP0 promotes transcription from viral genes, disrupts structures in the nucleus known as nuclear dots or promyelocytic leukemia (PML) nuclear bodies, and alters the expression of host and viral genes in combination with a neuron specific protein. At later stages of cellular infection, ICP0 relocates to the cell cytoplasm to be incorporated into new virion particles.

Herpes simplex virus1 and 2, also known by their taxonomical names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of new viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are common and contagious. They can be spread when an infected person begins shedding the virus.
Human betaherpesvirus 5, also called human cytomegalovirus (HCMV), is the type species of the virus genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals.
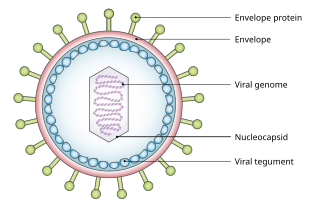
A viral tegument or tegument, more commonly known as a viral matrix, is a cluster of proteins that lines the space between the envelope and nucleocapsid of all herpesviruses. The tegument generally contains proteins that aid in viral DNA replication and evasion of the immune response, typically with inhibition of signalling in the immune system and activation of interferons. The tegument is usually released shortly after infection into the cytoplasm. These proteins are usually formed within the late phase of the viral infectious cycle, after viral genes have been replicated. Much information regarding viral teguments has been gathered from studying Herpes simplex virus.
Intrinsic immunity refers to a set of recently discovered cellular-based anti-viral defense mechanisms, notably genetically encoded proteins which specifically target eukaryotic retroviruses. Unlike adaptive and innate immunity effectors, intrinsic immune proteins are usually expressed at a constant level, allowing a viral infection to be halted quickly. Intrinsic antiviral immunity refers to a form of innate immunity that directly restricts viral replication and assembly, thereby rendering a cell non-permissive to a specific class or species of viruses. Intrinsic immunity is conferred by restriction factors preexisting in certain cell types, although these factors can be further induced by virus infection. Intrinsic viral restriction factors recognize specific viral components, but unlike other pattern recognition receptors that inhibit viral infection indirectly by inducing interferons and other antiviral molecules, intrinsic antiviral factors block viral replication immediately and directly.
Immunoevasins are proteins expressed by some viruses that enable the virus to evade immune recognition by preventing the appearance of peptide on MHC I complexes on the infected cell. Some viral immunoevasins block peptide entry into the endoplasmic reticulum (ER) by targeting the TAP transporters.
Herpes simplex research includes all medical research that attempts to prevent, treat, or cure herpes, as well as fundamental research about the nature of herpes. Examples of particular herpes research include drug development, vaccines and genome editing. HSV-1 and HSV-2 are commonly thought of as oral and genital herpes respectively, but other members in the herpes family include chickenpox (varicella/zoster), cytomegalovirus, and Epstein-Barr virus. There are many more members that infect animals other than humans, some of which cause disease in companion animals or have economic impacts in the agriculture industry.
HIV is commonly transmitted via unprotected sexual activity, blood transfusions, hypodermic needles, and from mother to child. Upon acquisition of the virus, the virus replicates inside and kills T helper cells, which are required for almost all adaptive immune responses. There is an initial period of influenza-like illness, and then a latent, asymptomatic phase. When the CD4 lymphocyte count falls below 200 cells/ml of blood, the HIV host has progressed to AIDS, a condition characterized by deficiency in cell-mediated immunity and the resulting increased susceptibility to opportunistic infections and certain forms of cancer.

Talimogene laherparepvec is a biopharmaceutical drug to treat melanoma that cannot be operated on; it is injected directly into a subset of lesions which generates a systemic immune response against the patient's cancer. The final four year analysis from the pivotal phase 3 study upon which TVEC was approved by the FDA showed a 31.5% response rate with a 16.9% CR rate. There was also a substantial and statistically significant survival benefit in patients with earlier metastatic disease and in patients who hadn't received prior systemic treatment for melanoma. The earlier stage group had a reduction in the risk of death of approximately 50% with one in four patients appearing to have met, or be close to be reaching, the medical definition of cure. Real world use of talimogene laherparepvec have shown response rates of up to 88.5% with CR rates of up to 61.5%.
ICP8, the herpes simplex virus type-1 single-strand DNA-binding protein, is one of seven proteins encoded in the viral genome of HSV-1 that is required for HSV-1 DNA replication. It is able to anneal to single-stranded DNA (ssDNA) as well as melt small fragments of double-stranded DNA (dsDNA); its role is to destabilize duplex DNA during initiation of replication. It differs from helicases because it is ATP- and Mg2+-independent. In cells infected with HSV-1, the DNA in those cells become colocalized with ICP8.

Many variants of herpes simplex virus have been considered for viral therapy of cancer; the early development of these was thoroughly reviewed in the journal Cancer Gene Therapy in 2002. This page describes the most notable variants—those tested in clinical trials: G207, HSV1716, NV1020 and Talimogene laherparepvec. These attenuated versions are constructed by deleting viral genes required for infecting or replicating inside normal cells but not cancer cells, such as ICP34.5, ICP6/UL39, and ICP47.
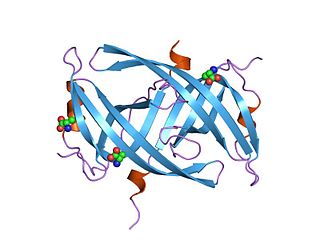
Single-stranded binding proteins (SSBs) are a class of proteins that have been identified in both viruses and organisms from bacteria to humans.
David M. Knipe is the Higgins Professor of Microbiology and Molecular Genetics and interim Co-Chair in the Department of Microbiology and Immunobiology at the Harvard Medical School in Boston, Massachusetts and co-chief editor of the reference book Fields Virology. He had previously served as Chair of the Program in Virology at Harvard Medical School from 2004 through 2016.
Infected cell protein 34.5 is a protein expressed by the ɣ34.5 gene in viruses such as herpes simplex virus; it blocks a cellular stress response to viral infection. It shares the C-terminal regulatory domain with protein phosphatase 1 subunit 15A/B.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.
References
- 1 2 Goldsmith K, Chen W, Johnson DC, Hendricks RL (February 1998). "Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response". The Journal of Experimental Medicine. 187 (3): 341–8. CiteSeerX 10.1.1.282.6406 . doi:10.1084/jem.187.3.341. PMC 2212130 . PMID 9449714.
- ↑ Berger C, Xuereb S, Johnson DC, Watanabe KS, Kiem HP, Greenberg PD, Riddell SR (May 2000). "Expression of herpes simplex virus ICP47 and human cytomegalovirus US11 prevents recognition of transgene products by CD8(+) cytotoxic T lymphocytes". Journal of Virology. 74 (10): 4465–73. doi:10.1128/jvi.74.10.4465-4473.2000. PMC 111967 . PMID 10775582.
| This virus-related article is a stub. You can help Wikipedia by expanding it. |