
Apoptosis is a form of programmed cell death that occurs in multicellular organisms and in some eukaryotic, single-celled microorganisms such as yeast. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, each day the approximate lost is 20 to 30 billion cells.

Baculoviridae is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera.

The apoptosome is a large quaternary protein structure formed in the process of apoptosis. Its formation is triggered by the release of cytochrome c from the mitochondria in response to an internal (intrinsic) or external (extrinsic) cell death stimulus. Stimuli can vary from DNA damage and viral infection to developmental cues such as those leading to the degradation of a tadpole's tail.

The BH3 interacting-domain death agonist, or BID, gene is a pro-apoptotic member of the Bcl-2 protein family. Bcl-2 family members share one or more of the four characteristic domains of homology entitled the Bcl-2 homology (BH) domains, and can form hetero- or homodimers. Bcl-2 proteins act as anti- or pro-apoptotic regulators that are involved in a wide variety of cellular activities.

Caspase-9 is an enzyme that in humans is encoded by the CASP9 gene. It is an initiator caspase, critical to the apoptotic pathway found in many tissues. Caspase-9 homologs have been identified in all mammals for which they are known to exist, such as Mus musculus and Pan troglodytes.

Caspase-8 is a caspase protein, encoded by the CASP8 gene. It most likely acts upon caspase-3. CASP8 orthologs have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also present in birds.
Inhibitors of apoptosis are a group of proteins that mainly act on the intrinsic pathway that block programmed cell death, which can frequently lead to cancer or other effects for the cell if mutated or improperly regulated. Many of these inhibitors act to block caspases, a family of cysteine proteases that play an integral role in apoptosis. Some of these inhibitors include the Bcl-2 family, viral inhibitor crmA, and IAP's.
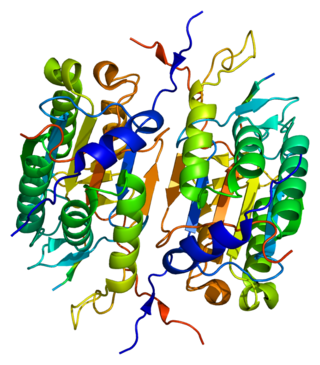
Caspase 2 also known as CASP2 is an enzyme that, in humans, is encoded by the CASP2 gene. CASP2 orthologs have been identified in nearly all mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts.

X-linked inhibitor of apoptosis protein (XIAP), also known as inhibitor of apoptosis protein 3 (IAP3) and baculoviral IAP repeat-containing protein 4 (BIRC4), is a protein that stops apoptotic cell death. In humans, this protein (XIAP) is produced by a gene named XIAP gene located on the X chromosome.

Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the CASP3 gene. CASP3 orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts.

Caspase-7, apoptosis-related cysteine peptidase, also known as CASP7, is a human protein encoded by the CASP7 gene. CASP7 orthologs have been identified in nearly all mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts.

Caspase-6 is an enzyme that in humans is encoded by the CASP6 gene. CASP6 orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts. Caspase-6 has known functions in apoptosis, early immune response and neurodegeneration in Huntington's and Alzheimer's disease.

Caspase-10 is an enzyme that, in humans, is encoded by the CASP10 gene.

Diablo homolog (DIABLO) is a mitochondrial protein that in humans is encoded by the DIABLO gene on chromosome 12. DIABLO is also referred to as second mitochondria-derived activator of caspases or SMAC. This protein binds inhibitor of apoptosis proteins (IAPs), thus freeing caspases to activate apoptosis. Due to its proapoptotic function, SMAC is implicated in a broad spectrum of tumors, and small molecule SMAC mimetics have been developed to enhance current cancer treatments.

Apoptotic protease activating factor 1, also known as APAF1, is a human homolog of C. elegans CED-4 gene.

Serine protease HTRA2, mitochondrial is an enzyme that in humans is encoded by the HTRA2 gene. This protein is involved in caspase-dependent apoptosis and in Parkinson's disease.

Caspase-activated DNase (CAD) or DNA fragmentation factor subunit beta is a protein that in humans is encoded by the DFFB gene. It breaks up the DNA during apoptosis and promotes cell differentiation. It is usually an inactive monomer inhibited by ICAD. This is cleaved before dimerization.

Picornain 3C is a protease found in picornaviruses, which cleaves peptide bonds of non-terminal sequences. Picornain 3C’s endopeptidase activity is primarily responsible for the catalytic process of selectively cleaving Gln-Gly bonds in the polyprotein of poliovirus and with substitution of Glu for Gln, and Ser or Thr for Gly in other picornaviruses. Picornain 3C are cysteine proteases related by amino acid sequence to trypsin-like serine proteases. Picornain 3C is encoded by enteroviruses, rhinoviruses, aphtoviruses and cardioviruses. These genera of picoviruses cause a wide range of infections in humans and mammals.

The protein Sf caspase-1 is the insect ortholog of the human effector caspases CASP3 (CPP32) and CASP7 (MCH3) in the species Spodoptera frugiperda. It was identified as the target of the baculoviral caspase inhibitor protein P35, which it cleaves and by which it is inhibited. Like other caspases, Sf caspase-1 is an aspartate-specific cysteine protease that is produced as an inactive proenzyme and becomes activated by autocatalytic cleavage. The Sf caspase-1 proenzyme is cleaved after the amino acid residues Asp-28 and Asp-195, resulting in a smaller 12 kDa fragment and a larger 19 kDa fragment. Just like with human caspases CASP3 or CASP7, the two cleavage fragments form heterodimers, which again form biologically active dimers-of-heterodimers consisting of two smaller and two larger fragments. Some experiments also showed cleavage of Sf caspase-1 at the residue Asp-184, resulting in an 18 kDa instead of 19 kDa fragment, however this result is likely an in vitro artefact. The insect immunophilin FKBP46 is a substrate of Sf caspase-1, which cleaves full length FKBP46 resulting in a ~25 kDa fragment.

Death regulator Nedd2-like caspase was firstly identified and characterised in Drosophila in 1999 as a cysteine protease containing an amino-terminal caspase recruitment domain. At first, it was thought of as an effector caspase involved in apoptosis, but subsequent findings have proved that it is, in fact, an initiator caspase with a crucial role in said type of programmed cell death.



















