
Apoptosis is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, D.N.A. fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day.

Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.
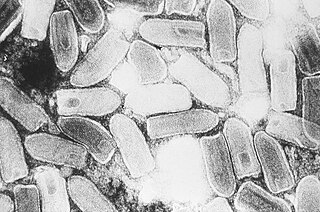
Indiana vesiculovirus, formerly Vesicular stomatitis Indiana virus is a virus in the family Rhabdoviridae; the well-known Rabies lyssavirus belongs to the same family. VSIV can infect insects, cattle, horses and pigs. It has particular importance to farmers in certain regions of the world where it infects cattle. This is because its clinical presentation is identical to the very important foot and mouth disease virus.

The mumps virus (MuV) is the virus that causes mumps. MuV contains a single-stranded, negative-sense genome made of ribonucleic acid (RNA). Its genome is about 15,000 nucleotides in length and contains seven genes that encode nine proteins. The genome is encased by a capsid that is in turn surrounded by a viral envelope. MuV particles, called virions, are pleomorphic in shape and vary in size from 100 to 600 nanometers in diameter. One serotype and twelve genotypes that vary in their geographic distribution are recognized. Humans are the only natural host of the mumps virus.

Rubella virus (RuV) is the pathogenic agent of the disease rubella, transmitted only between humans via the respiratory route, and is the main cause of congenital rubella syndrome when infection occurs during the first weeks of pregnancy.
Microviridae is a family of bacteriophages with a single-stranded DNA genome. The name of this family is derived from the ancient Greek word μικρός (mikrós), meaning "small". This refers to the size of their genomes, which are among the smallest of the DNA viruses. Enterobacteria, intracellular parasitic bacteria, and spiroplasma serve as natural hosts. There are 22 species in this family, divided among seven genera and two subfamilies.

Baculoviridae is a family of viruses. Arthropods, Lepidoptera, Hymenoptera, and Diptera serve as natural hosts. Currently, 85 species in are placed this family, assigned to four genera.

The M1 protein is a matrix protein of the influenza virus. It forms a coat inside the viral envelope. This is a bifunctional membrane/RNA-binding protein that mediates the encapsidation of RNA-nucleoprotein cores into the membrane envelope. It is therefore required that M1 binds both membrane and RNA simultaneously.

Murine respirovirus, formerly Sendai virus (SeV) and previously also known as murine parainfluenza virus type 1 or hemagglutinating virus of Japan (HVJ), is an enveloped,150-200 nm in diameter, a negative sense, single-stranded RNA virus of the family Paramyxoviridae. It typically infects rodents and it is not pathogenic for humans or domestic animals. Sendai virus (SeV) is a member of genus Respirovirus. The virus was isolated in the city of Sendai in Japan in the early 1950s. Since then, it has been actively used in research as a model pathogen. The virus is infectious for many cancer cell lines, has oncolytic properties demonstrated in animal models and in naturally-occurring cancers in animals. SeV's ability to fuse eukaryotic cells and to form syncytium was used to produce hybridoma cells capable of manufacturing monoclonal antibodies in large quantities. Recent applications of SeV-based vectors include the reprogramming of somatic cells into induced pluripotent stem cells and vaccines creation. For vaccination purpose the Sendai virus-based constructs could be delivered in a form of nasal drops, which may be beneficial in inducing a mucosal immune response. SeV has several features that are important in a vector for a successful vaccine: the virus does not integrate into the host genome, it does not undergo genetic recombination, it replicates only in the cytoplasm without DNA intermediates or a nuclear phase and it is not causing any disease in humans or domestic animals. Sendai virus is used as a backbone for vaccine development against Mycobacterium tuberculosis that causes tuberculosis, against HIV-1 that causes AIDS and against respiratory syncytial virus (RSV) that causes respiratory infection in children. The vaccine development against Mycobacterium tuberculosis is in pre-clinical stage, against HIV-1 it reached phase II clinical trial and against RSV it is in phase I. Fudan University in collaboration with ID Pharma Co. Ltd. is engaged in development of the vaccine for COVID-19 prevention. SeV serves as a vaccine backbone vector in the project.

Myxoma virus is a poxvirus in the genus Leporipoxvirus. There are two broad geographic types of Myxoma virus, Californian and South American. Californian myxoma virus is found on the west coast of the United States, the Baja peninsula of Mexico, and the southwest coast of Canada. South American or Brazilian myxoma virus is found in South and Central America. South American myxoma virus circulates in the jungle rabbit or tapeti, whereas Californian myxoma virus circulates in the brush rabbit. In their native hosts, the viruses cause the formation of benign cutaneous fibromas rather than systemic disease.

An alveolar macrophage, pulmonary macrophage, is a type of macrophage, a professional phagocyte, found in the airways and at the level of the alveoli in the lungs, but separated from their walls.

Mitochondrial antiviral-signaling protein (MAVS) is a protein that is essential for antiviral innate immunity. MAVS is located in the inner membrane of the mitochondria, peroxisomes, and endoplasmic reticulum (ER). Upon viral infection, a group of cytosolic proteins will detect the presence of the virus and bind to MAVS, thereby activating MAVS. The activation of MAVS leads the virally infected cell to secrete cytokines. This induces an immune response which kills the host's virally infected cells, resulting in clearance of the virus.
Pseudotyping is the process of producing viruses or viral vectors in combination with foreign viral envelope proteins. The result is a pseudotyped virus particle, also called a pseudovirus. With this method, the foreign viral envelope proteins can be used to alter host tropism or increase or decrease the stability of the virus particles. Pseudotyped particles do not carry the genetic material to produce additional viral envelope proteins, so the phenotypic changes cannot be passed on to progeny viral particles. In some cases, the inability to produce viral envelope proteins renders the pseudovirus replication incompetent. In this way, the properties of dangerous viruses can be studied in a lower risk setting.

Herpesvirus glycoprotein B is a viral glycoprotein that is involved in the viral cell entry of Herpes simplex virus (HSV). Herpesviruses have a lipid bilayer, called the envelope, which contains twelve surface glycoproteins. For infectivity to be attained, the double stranded DNA genome of HSV must enter the host cell through means of fusion of its envelope with the cellular membrane or via endocytosis. Other viral glycoproteins involved in the process of viral cell entry include gC, gB, gD, gH, and gL, but only gC, gB, gD, and gH are required for the fusion of the HSV's envelope with the cellular membrane. It can be noted that all herpesviruses have glycoproteins gB, gH, and gL.
Retroviral matrix proteins are components of envelope-associated capsids of retroviruses. These proteins line the inner surface of viral envelopes and are associated with viral membranes.
Avian orthoreovirus, also known as avian reovirus, is an orthoreovirus from the Reoviridae family. Infection causes arthritis and tenosynovitis in poultry. It can also cause respiratory disease.

In molecular biology, VP40 is the name of a viral matrix protein. Most commonly it is found in the Ebola virus (EBOV), a type of non-segmented, negative-strand RNA virus. Ebola virus causes a severe and often fatal haemorrhagic fever in humans, known as Ebola virus disease. The virus matrix protein VP40 is a major structural protein that plays a central role in virus assembly and budding at the plasma membrane of infected cells. VP40 proteins work by associating with cellular membranes, interacting with the cytoplasmic tails of glycoproteins and binding to the ribonucleoprotein complex.

Picornain 3C is a protease found in picornaviruses, which cleaves peptide bonds of non-terminal sequences. Picornain 3C’s endopeptidase activity is primarily responsible for the catalytic process of selectively cleaving Gln-Gly bonds in the polyprotein of poliovirus and with substitution of Glu for Gln, and Ser or Thr for Gly in other picornaviruses. Picornain 3C are cysteine proteases related by amino acid sequence to trypsin-like serine proteases. Picornain 3C is encoded by enteroviruses, rhinoviruses, aphtoviruses and cardioviruses. These genera of picoviruses cause a wide range of infections in humans and mammals.
David M. Knipe is the Higgins Professor of Microbiology and Molecular Genetics in the Department of Microbiology at the Harvard Medical School in Boston, Massachusetts and co-chief editor of the reference book Fields Virology. He returned to the Chair of the Program in Virology at Harvard Medical School in 2019, having previously held the position from 2004 through 2016 and served as interim Co-Chair of the Microbiology and Immunobiology Department from 2016 through 2018.

The Early 35 kDa protein, or P35 in short, is a baculoviral protein that inhibits apoptosis in the cells infected by the virus. Although baculoviruses infect only invertebrates in nature, ectopic expression of P35 in vertebrate animals and cells also results in inhibition of apoptosis, thus indicating a universal mechanism. P35 has been shown to be a caspase inhibitor with a very wide spectrum of activity both in regard to inhibited caspase types and to species in which the mechanism is conserved.















