Related Research Articles
An emulsion is a mixture of two or more liquids that are normally immiscible owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid is dispersed in the other. Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working.

Surfactants are molecules that spontaneously bond with each other to form sealed bubbles. Surfactants are compounds that lower the surface tension between two liquids, between a gas and a liquid, or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants.
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.
Sodium stearoyl-2-lactylate is a versatile, FDA approved food additive used to improve the mix tolerance and volume of processed foods. It is one type of a commercially available lactylate. SSL is non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Because SSL is a safe and highly effective food additive, it is used in a wide variety of products ranging from baked goods and desserts to pet foods.

Easy Cheese is the trademark for a processed cheese spread product distributed by Mondelēz International. It is also referred to as aerosol cheese, spray cheese or simply cheese in a can, and is similar to squeeze cheese. Easy Cheese is packaged in a metal can filled with air covered with a plastic cap that reveals a straight, flexible nozzle where the cheese is extruded.

A thickening agent or thickener is a substance which can increase the viscosity of a liquid without substantially changing its other properties. Edible thickeners are commonly used to thicken sauces, soups, and puddings without altering their taste; thickeners are also used in paints, inks, explosives, and cosmetics.

Charcuterie is a French term for a branch of cooking devoted to prepared meat products, such as bacon, ham, sausage, terrines, galantines, ballotines, pâtés, and confit, primarily from pork.

Carboxymethyl cellulose (CMC) or cellulose gum is a cellulose derivative with carboxymethyl groups (-CH2-COOH) bound to some of the hydroxyl groups of the glucopyranose monomers that make up the cellulose backbone. It is often used as its sodium salt, sodium carboxymethyl cellulose. It used to be marketed under the name Tylose, a registered trademark of SE Tylose.

A cream is a preparation usually for application to the skin. Creams for application to mucous membranes such as those of the rectum or vagina are also used. Creams may be considered pharmaceutical products as even cosmetic creams are based on techniques developed by pharmacy and unmedicated creams are highly used in a variety of skin conditions (dermatoses). The use of the finger tip unit concept may be helpful in guiding how much topical cream is required to cover different areas.
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
The Bancroft rule states: "The phase in which an emulsifier is more soluble constitutes the continuous phase."

Water-in-water (W/W) emulsion is a system that consists of droplets of water-solvated molecules in another continuous aqueous solution; both the droplet and continuous phases contain different molecules that are entirely water-soluble. As such, when two entirely aqueous solutions containing different water-soluble molecules are mixed, water droplets containing predominantly one component are dispersed in water solution containing another component. Recently, such a water-in-water emulsion was demonstrated to exist and be stable from coalescence by the separation of different types of non-amphiphilic, but water-soluble molecular interactions. These molecular interactions include hydrogen bonding, pi stacking, and salt bridging. This w/w emulsion was generated when the different water-solvated molecular functional groups get segregated in an aqueous mixture consisting of polymer and liquid crystal molecules.

The ouzo effect is a cloudy (louche) oil-in-water emulsion that is formed when water is added to ouzo and other anise-flavored liqueurs and spirits, such as absinthe, arak, limoncello, pastis, rakı, sambuca, and tsipouro. Such microemulsions occur with only minimal mixing and are highly stable.
Creaming, in the laboratory sense, is the migration of the dispersed phase of an emulsion, under the influence of buoyancy. The particles float upwards or sink, depending on how large they are and how much less dense or more dense they may be than the continuous phase, and also how viscous or how thixotropic the continuous phase might be. For as long as the particles remain separated, the process is called creaming.
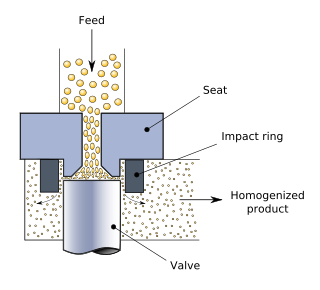
Homogenization or homogenisation is any of several processes used to make a mixture of two mutually non-soluble liquids the same throughout. This is achieved by turning one of the liquids into a state consisting of extremely small particles distributed uniformly throughout the other liquid. A typical example is the homogenization of milk, where the milk fat globules are reduced in size and dispersed uniformly through the rest of the milk.

Membrane emulsification (ME) is a relatively novel technique for producing all types of single and multiple emulsions for DDS, solid micro carriers for encapsulation of drug or nutrient, solder particles for surface-mount technology, mono dispersed polymer microspheres. Membrane emulsification was introduced by Nakashima and Shimizu in the late 1980s in Japan.
Emulsified Fuels are emulsions composed of water and a combustible liquid, either oil or a fuel. Emulsions are a particular example of a dispersion comprising a continuous and a dispersed phase. Most commonly used emulsion fuel is water-in-diesel emulsion. In the case of emulsions both phases are the immiscible liquids, oil and water. Emulsion fuels can be either a microemulsion or an ordinary emulsion. The essential differences between the two are stability and particle size distribution. Microemulsions are isotropic whereas macroemulsions are prone to settling and changes in particle size over time. Both use surfactants and can be either water-in-oil, or oil-in-water or bicontinuous.
Lactylates are organic compounds that are FDA approved for use as food additives and cosmetic ingredients. These additives are non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Owing to their safety and versatile functionality, lactylates are used in a wide variety of food and non-food applications. In the United States, the Food Chemicals Codex specifies the labeling requirements for food ingredients including lactylates. In the European Union, lactylates must be labelled in accordance with the requirements of the applicable EU regulation. Lactylates may be labelled as calcium stearoyl lactylate (CSL), sodium stearoyl lactylate (SSL), or lactylic esters of fatty acids (LEFA).
Calcium stearoyl-2-lactylate or E482 is a versatile, FDA approved food additive. It is one type of a commercially available lactylate. CSL is non-toxic, biodegradable, and typically manufactured using biorenewable feedstocks. Because CSL is a safe and highly effective food additive, it is used in a wide variety of products from baked goods and desserts to packaging.
Macroemulsions are homogenous transparent thermodynamically unstable systems with particle sizes ranging from 5-140 nm, which form spontaneously when mixed in the correct ratio. Macroemulsions scatter light effectively and therefore appear milky, because their droplets are greater than a wavelength of light. They are part of a larger family of emulsions along with microemulsions. As with all emulsions, one phase serves as the dispersing agent. It is often called the continuous or outer phase. The remaining phase(s) are disperse or inner phase(s), because the liquid droplets are finely distributed amongst the larger continuous phase droplets. This type of emulsion is thermodynamically unstable, but can be stabilized for a period of time with applications of kinetic energy. Surfactants (emulsifiers) are used to reduce the interfacial tension between the two layers, and induce macroemulsion stability for a useful amount of time.
References
- ↑ Knipe, C. Lynn (1987). "Meat Emulsions | Meat Science Extension". meatsci.osu.edu. Retrieved 2018-04-08.
- ↑ Owusu-Apenten, R.K. (2004). "10 - Testing protein functionality". Science Direct. Woodhead Publishing. pp. 217–244. doi:10.1533/9781855738379.2.217 . Retrieved 2020-11-23.
- ↑ Elton D. Aberle; et al. (2001). Principles of Meat Science. Kendall Hunt. pp. 126–144. ISBN 9780787247201 . Retrieved 2 February 2017.