An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorder and its related psychological and physical symptoms.

Dysautonomia or autonomic dysfunction is a condition in which the autonomic nervous system (ANS) does not work properly. This may affect the functioning of the heart, bladder, intestines, sweat glands, pupils, and blood vessels. Dysautonomia has many causes, not all of which may be classified as neuropathic. A number of conditions can feature dysautonomia, such as Parkinson's disease, multiple system atrophy, and dementia with Lewy bodies, Ehlers-Danlos syndromes, autoimmune autonomic ganglionopathy and autonomic neuropathy, HIV/AIDS, autonomic failure, and postural orthostatic tachycardia syndrome.

Phenylpiracetam, is a phenylated analog of the drug piracetam. It was developed in 1983 as a medication for Soviet Cosmonauts to treat the prolonged stresses of working in space. Phenylpiracetam was created at the Russian Academy of Sciences Institute of Biomedical Problems in an effort led by psychopharmacologist Valentina Ivanovna Akhapkina. In Russia it is now available as a prescription drug. Research on animals has indicated that phenylpiracetam may have anti-amnesic, antidepressant, anticonvulsant, anxiolytic, and memory enhancement effects.

Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate dopamine-related activity. For example, certain proteins such as the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and dopamine receptors can be classified as dopaminergic, and neurons that synthesize or contain dopamine and synapses with dopamine receptors in them may also be labeled as dopaminergic. Enzymes that regulate the biosynthesis or metabolism of dopamine such as aromatic L-amino acid decarboxylase or DOPA decarboxylase, monoamine oxidase (MAO), and catechol O-methyl transferase (COMT) may be referred to as dopaminergic as well. Also, any endogenous or exogenous chemical substance that acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons that synapse onto neurons that release dopamine or express dopamine receptors) can also be said to have dopaminergic effects, two prominent examples being opioids, which enhance dopamine release indirectly in the reward pathways, and some substituted amphetamines, which enhance dopamine release directly by binding to and inhibiting VMAT2.

Tianeptine, sold under the brand names Stablon and Coaxil among others, is an atypical antidepressant which is used mainly in the treatment of major depressive disorder, although it may also be used to treat anxiety, asthma, and irritable bowel syndrome.

Piribedil (trade names Pronoran, Trivastal Retard, Trastal, Trivastan, Clarium and others) is an antiparkinsonian agent and piperazine derivative which acts as a D2 and D3 receptor agonist. It also has α2-adrenergic antagonist properties.
Oneiroid syndrome (OS) is a condition involving dream-like disturbances of one's consciousness by vivid scenic hallucinations, accompanied by catatonic symptoms, delusions, or psychopathological experiences of a kaleidoscopic nature. The term is from Ancient Greek "ὄνειρος" and "εἶδος". It is a common complication of catatonic schizophrenia, although it can also be caused by other mental disorders. The dream-like experiences are vivid enough to seem real to the patient. OS is distinguished from delirium by the fact that the imaginative experiences of patients always have an internal projection. This syndrome is hardly mentioned in standard psychiatric textbooks, possibly because it is not listed in DSM.
Mesocarb is a drug that is currently being developed for Parkinson's disease.

Periciazine (INN), also known as pericyazine (BAN) or propericiazine, is a drug that belongs to the phenothiazine class of typical antipsychotics.
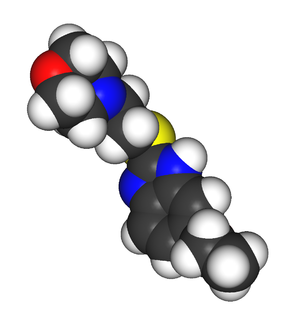
Fabomotizole is an anxiolytic drug launched in Russia in the early 2000s. It produces anxiolytic and neuroprotective effects without any sedative or muscle relaxant actions. Its mechanism of action remains poorly defined however, with GABAergic, NGF- and BDNF-release-promoting, MT1 receptor agonism, MT3 receptor antagonism, and sigma agonism suggested as potential mechanisms. Fabomotizole was shown to inhibit MAO-A reversibly and there might be also some involvement with serotonin receptors. Clinical trials have shown fabomotizole to be well tolerated and reasonably effective for the treatment of anxiety.

Selank is a nootropic, anxiolytic peptide based drug developed by the Institute of Molecular Genetics of the Russian Academy of Sciences. Selank is a heptapeptide with the sequence Thr-Lys-Pro-Arg-Pro-Gly-Pro (TKPRPGP). It is a synthetic analogue of human tuftsin.

Pipofezine, sold under the brand name Azafen or Azaphen, is an antidepressant approved in Russia for the treatment of depression. It was introduced in the late 1960s and is still used today.
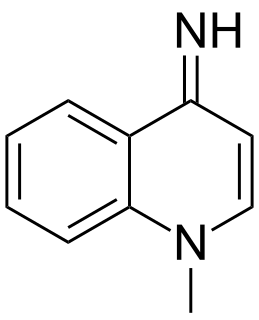
Echinopsidine (Adepren) is an antidepressant that was under development in Bulgaria for the treatment of depression. It increases serotonin, norepinephrine, and dopamine levels in the brain and is believed to act as a monoamine oxidase inhibitor (MAOI). Echinopsidine is found naturally in Echinops echinatus along with the related alkaloids echinopsine and echinozolinone.

Mepiprazole is an anxiolytic drug of the phenylpiperazine group with additional antidepressant properties that is marketed in Spain. It acts as a 5-HT2A and α1-adrenergic receptor antagonist and inhibits the reuptake and induces the release of serotonin, dopamine, and norepinephrine to varying extents, and has been described as a serotonin antagonist and reuptake inhibitor (SARI). Controlled clinical trials of mepiprazole in patients with irritable bowel syndrome (IBS) were also carried out and suggested some benefits of the drug in relieving symptoms of IBS in some patients. Similarly to other phenylpiperazines like trazodone, nefazodone, and etoperidone, mepiprazole produces mCPP as an active metabolite.

Emoxypine (2-ethyl-6-methyl-3-hydroxypyridine), also known as Mexidol or Mexifin when used as the succinate salt, is an antioxidant manufactured in Russia by Pharmasoft Pharmaceuticals. Its chemical structure resembles that of pyridoxine (a type of vitamin B6). Being a Russian company, they did not seek approval for any medical use in the United States or Europe; similarly, US and EU companies do not seek approval for any medical use in Russia.
A somatic symptom disorder, formerly known as a somatoform disorder, is any mental disorder that manifests as physical symptoms that suggest illness or injury, but cannot be explained fully by a general medical condition or by the direct effect of a substance, and are not attributable to another mental disorder. Somatic symptom disorders, as a group, are included in a number of diagnostic schemes of mental illness, including the Diagnostic and Statistical Manual of Mental Disorders.
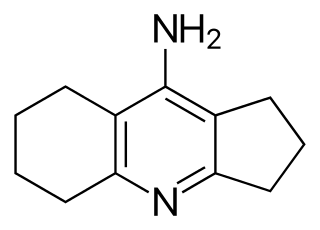
Ipidacrine (Neiromidin) is a drug first synthesized by the National Research Center for Biologically Active Compounds in the Russian Federation. This compound is a ring-constricted derivative of tacrine (Cognex).

Metadoxine, also known as pyridoxine-pyrrolidone carboxylate, is a drug used to treat chronic and acute alcohol intoxication. Metadoxine accelerates alcohol clearance from the blood.

Brilaroxazine, also known by the developmental code names RP-5063 and RP-5000, is an investigational atypical antipsychotic which is under development by Reviva Pharmaceuticals for the treatment of schizophrenia and schizoaffective disorder. Reviva Pharmaceuticals also intends to investigate brilaroxazine for the treatment of bipolar disorder, major depressive disorder, psychosis/agitation associated with Alzheimer's disease, Parkinson's disease psychosis, attention deficit hyperactivity disorder (ADD/ADHD), and autism. As of May 2015, it is in phase III clinical trials for schizophrenia.















