
A blood cell, also called a hematopoietic cell, hemocyte, or hematocyte, is a cell produced through hematopoiesis and found mainly in the blood. Major types of blood cells include red blood cells (erythrocytes), white blood cells (leukocytes), and platelets (thrombocytes). Together, these three kinds of blood cells add up to a total 45% of the blood tissue by volume, with the remaining 55% of the volume composed of plasma, the liquid component of blood.
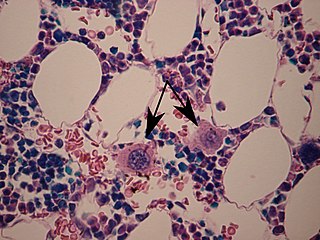
A megakaryocyte is a large bone marrow cell with a lobated nucleus that produces blood platelets (thrombocytes), which are necessary for normal clotting. In humans, megakaryocytes usually account for 1 out of 10,000 bone marrow cells, but can increase in number nearly 10-fold during the course of certain diseases. Owing to variations in combining forms and spelling, synonyms include megalokaryocyte and megacaryocyte.

Granulocytes are cells in the innate immune system characterized by the presence of specific granules in their cytoplasm. Such granules distinguish them from the various agranulocytes. All myeloblastic granulocytes are polymorphonuclear, that is, they have varying shapes (morphology) of the nucleus ; and are referred to as polymorphonuclear leukocytes. In common terms, polymorphonuclear granulocyte refers specifically to "neutrophil granulocytes", the most abundant of the granulocytes; the other types have varying morphology. Granulocytes are produced via granulopoiesis in the bone marrow.

Erythropoiesis is the process which produces red blood cells (erythrocytes), which is the development from erythropoietic stem cell to mature red blood cell.
Primary myelofibrosis (PMF) is a rare bone marrow blood cancer. It is classified by the World Health Organization (WHO) as a type of myeloproliferative neoplasm, a group of cancers in which there is activation and growth of mutated cells in the bone marrow. This is most often associated with a somatic mutation in the JAK2, CALR, or MPL genes. In PMF, the bony aspects of bone marrow are remodeled in a process called osteosclerosis; in addition, fibroblast secrete collagen and reticulin proteins that are collectively referred to as (fibrosis). These two pathological processes compromise the normal function of bone marrow resulting in decreased production of blood cells such as erythrocytes, granulocytes and megakaryocytes, the latter cells responsible for the production of platelets.

The myeloblast is a unipotent stem cell which differentiates into the effectors of the granulocyte series. It is found in the bone marrow. Stimulation of myeloblasts by G-CSF and other cytokines triggers maturation, differentiation, proliferation and cell survival.
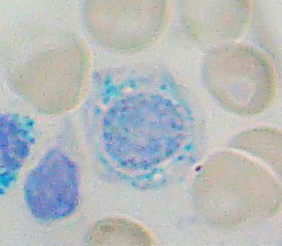
Sideroblastic anemia, or sideroachrestic anemia, is a form of anemia in which the bone marrow produces ringed sideroblasts rather than healthy red blood cells (erythrocytes). In sideroblastic anemia, the body has iron available but cannot incorporate it into hemoglobin, which red blood cells need in order to transport oxygen efficiently. The disorder may be caused either by a genetic disorder or indirectly as part of myelodysplastic syndrome, which can develop into hematological malignancies.

A proerythroblast is the earliest of four stages in development of the normoblast.

A myelocyte is a young cell of the granulocytic series, occurring normally in bone marrow.

GATA-binding factor 1 or GATA-1 is the founding member of the GATA family of transcription factors. This protein is widely expressed throughout vertebrate species. In humans and mice, it is encoded by the GATA1 and Gata1 genes, respectively. These genes are located on the X chromosome in both species.

Monoblasts are the committed progenitor cells that differentiated from a committed macrophage or dendritic cell precursor (MDP) in the process of hematopoiesis. They are the first developmental stage in the monocyte series leading to a macrophage. Their myeloid cell fate is induced by the concentration of cytokines they are surrounded by during development. These cytokines induce the activation of transcription factors which push completion of the monoblast's myeloid cell fate. Monoblasts are normally found in bone marrow and do not appear in the normal peripheral blood. They mature into monocytes which, in turn, develop into macrophages. They then are seen as macrophages in the normal peripheral blood and many different tissues of the body. Macrophages can produce a variety of effector molecules that initiate local, systemic inflammatory responses. These monoblast differentiated cells are equipped to fight off foreign invaders using pattern recognition receptors to detect antigen as part of the innate immune response.

A promegakaryocyte is a precursor cell for a megakaryocyte. It arises from a megakaryoblast, into a promegakaryocyte and then into a megakaryocyte, which will eventually break off and become a platelet.

Toxic granulation refers to dark coarse granules found in granulocytes, particularly neutrophils, in patients with inflammatory conditions.

Acute megakaryoblastic leukemia (AMKL) is life-threatening leukemia in which malignant megakaryoblasts proliferate abnormally and injure various tissues. Megakaryoblasts are the most immature precursor cells in a platelet-forming lineage; they mature to promegakaryocytes and, ultimately, megakaryocytes which cells shed membrane-enclosed particles, i.e. platelets, into the circulation. Platelets are critical for the normal clotting of blood. While malignant megakaryoblasts usually are the predominant proliferating and tissue-damaging cells, their similarly malignant descendants, promegakaryocytes and megakaryocytes, are variable contributors to the malignancy.
In hematology, myelopoiesis in the broadest sense of the term is the production of bone marrow and of all cells that arise from it, namely, all blood cells. In a narrower sense, myelopoiesis also refers specifically to the regulated formation of myeloid leukocytes (myelocytes), including eosinophilic granulocytes, basophilic granulocytes, neutrophilic granulocytes, and monocytes.

CFU-GEMM is a colony forming unit that generates myeloid cells. CFU-GEMM cells are the oligopotential progenitor cells for myeloid cells; they are thus also called common myeloid progenitor cells or myeloid stem cells. "GEMM" stands for granulocyte, erythrocyte, monocyte, megakaryocyte.

Thrombopoiesis is the formation of thrombocytes in the bone marrow. Thrombopoietin is the main regulator of thrombopoiesis. Thrombopoietin affects most aspects of the production of platelets. This includes self-renewal and expansion of hematopoietic stem cells, stimulating the increase of megakaryocyte progenitor cells, and supporting these cells so they mature to become platelet-producing cells. The process of Thrombopoiesis is caused by the breakdown of proplatelets. During the process almost all of the membranes, organelles, granules, and soluble macromolecules in the cytoplasm are being consumed. Apoptosis also plays a role in the final stages of thrombopoiesis by letting proplatelet processes to occur from the cytoskeleton of actin.

White blood cells, also called immune cells or immunocytes, are cells of the immune system that are involved in protecting the body against both infectious disease and foreign invaders. White blood cells include three main subtypes: granulocytes, lymphocytes and monocytes.

A white blood cell differential is a medical laboratory test that provides information about the types and amounts of white blood cells in a person's blood. The test, which is usually ordered as part of a complete blood count (CBC), measures the amounts of the five normal white blood cell types – neutrophils, lymphocytes, monocytes, eosinophils and basophils – as well as abnormal cell types if they are present. These results are reported as percentages and absolute values, and compared against reference ranges to determine whether the values are normal, low, or high. Changes in the amounts of white blood cells can aid in the diagnosis of many health conditions, including viral, bacterial, and parasitic infections and blood disorders such as leukemia.
Transient myeloproliferative disease (TMD) occurs in a significant percentage of individuals born with the congenital genetic disorder, Down syndrome. It may occur in individuals who are not diagnosed with the syndrome but have some hematological cells containing genetic abnormalities that are similar to those found in Down syndrome. TMD usually develops in utero, is diagnosed prenatally or within ~3 months of birth, and thereafter resolves rapidly and spontaneously. However, during the prenatal-to-postnatal period, the disease may cause irreparable damage to various organs and in ~20% of individuals death. Moreover, ~10% of individuals diagnosed with TMD develop acute megakaryoblastic leukemia at some time during the 5 years following its resolution. TMD is a life-threatening, precancerous condition in fetuses as well as infants in their first few months of life.

















