
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradation occurs under a specific set of circumstances.

Bioremediation broadly refers to any process wherein a biological system, living or dead, is employed for removing environmental pollutants from air, water, soil, flue gasses, industrial effluents etc., in natural or artificial settings. The natural ability of organisms to adsorb, accumulate, and degrade common and emerging pollutants has attracted the use of biological resources in treatment of contaminated environment. In comparison to conventional physicochemical treatment methods bioremediation may offer advantages as it aims to be sustainable, eco-friendly, cheap, and scalable.
Biological augmentation is the addition of archaea or bacterial cultures required to speed up the rate of degradation of a contaminant. Organisms that originate from contaminated areas may already be able to break down waste, but perhaps inefficiently and slowly.
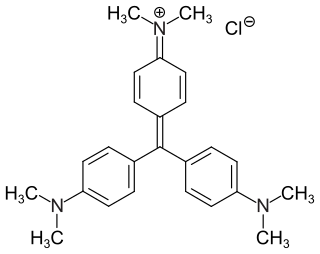
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each cycle of such interactions." In many cases, the catalyst is a solid that upon irradiation with UV- or visible light generates electron–hole pairs that generate free radicals. Photocatalysts belong to three main groups; heterogeneous, homogeneous, and plasmonic antenna-reactor catalysts. The use of each catalysts depends on the preferred application and required catalysis reaction.

Mycoremediation is a form of bioremediation in which fungi-based remediation methods are used to decontaminate the environment. Fungi have been proven to be a cheap, effective and environmentally sound way for removing a wide array of contaminants from damaged environments or wastewater. These contaminants include heavy metals, organic pollutants, textile dyes, leather tanning chemicals and wastewater, petroleum fuels, polycyclic aromatic hydrocarbons, pharmaceuticals and personal care products, pesticides and herbicides in land, fresh water, and marine environments.
Cometabolism is defined as the simultaneous degradation of two compounds, in which the degradation of the second compound depends on the presence of the first compound. This is in contrast to simultaneous catabolism, where each substrate is catabolized concomitantly by different enzymes. Cometabolism occurs when an enzyme produced by an organism to catalyze the degradation of its growth-substrate to derive energy and carbon from it is also capable of degrading additional compounds. The fortuitous degradation of these additional compounds does not support the growth of the bacteria, and some of these compounds can even be toxic in certain concentrations to the bacteria.
Silver molybdate (Ag2MoO4), a chemical compound, is a yellow, cubic crystalline substance often used in glass. Its crystals present two types of electronic structure, depending on the pressure conditions to which the crystal is subjected. At room temperature, Ag2MoO4 exhibits a spinel-type cubic structure, known as β-Ag2MoO4, which is more stable in nature. However, when exposed to high hydrostatic pressure, the tetragonal α-Ag2MoO4 forms as a metastable phase.
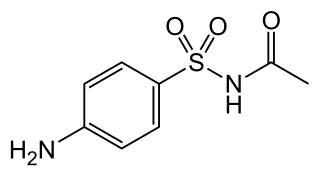
Sulfacetamide is a sulfonamide antibiotic commonly used in the treatment of bacterial infections, particularly those affecting the eyes and skin. It functions by inhibiting the synthesis of folic acid in bacteria, which is essential for their growth and reproduction, thereby exerting a bacteriostatic effect. Available in various forms, including eye drops, topical solutions, and creams, sulfacetamide is often prescribed for conditions such as conjunctivitis, seborrheic dermatitis, and acne vulgaris. Its efficacy, coupled with a relatively low risk of side effects, makes it a widely utilized agent in both ophthalmic and dermatologic care.

Diethyl phthalate (DEP) is a phthalate ester. It occurs as a colourless liquid without significant odour but has a bitter, disagreeable taste. It is more dense than water and insoluble in water; hence, it sinks in water.

Calliandra haematocephala, the red powder puff, is a species of flowering plants of the genus Calliandra in the family Fabaceae.
Biodegradable additives are additives that enhance the biodegradation of polymers by allowing microorganisms to utilize the carbon within the polymer chain as a source of energy. Biodegradable additives attract microorganisms to the polymer through quorum sensing after biofilm creation on the plastic product. Additives are generally in masterbatch formation that use carrier resins such as polyethylene (PE), polypropylene (PP), polystyrene (PS) or polyethylene terephthalate (PET).

The plastisphere is a human-made ecosystem consisting of organisms able to live on plastic waste. Plastic marine debris, most notably microplastics, accumulates in aquatic environments and serves as a habitat for various types of microorganisms, including bacteria and fungi. As of 2022, an estimated 51 trillion microplastics are floating in the surface water of the world's oceans. A single 5mm piece of plastic can host 1,000s of different microbial species. Some marine bacteria can break down plastic polymers and use the carbon as a source of energy.

Industrial dye degradation is any of a number of processed by which dyes are broken down, ideally into innocuous products. Many dyes, specifically in the textile industry such as methylene blue or methyl red, are released into ecosystems through water waste. Many of these dyes can be carcinogenic. In paper recycling dyes can be removed from fibres during a deinking stage prior to degradation.
Gordonia sp. nov. Q8 is a bacterium in the phylum of Actinomycetota. It was discovered in 2017 as one of eighteen new species isolated from the Jiangsu Wei5 oilfield in East China with the potential for bioremediation. Strain Q8 is rod-shaped and gram-positive with dimensions 1.0–4.0 μm × 0.5–1.2 μm and an optimal growth temperature of 40 °C. Phylogenetically, it is most closely related to Gordonia paraffinivorans and Gordonia alkaliphila, both of which are known bioremediators. Q8 was assigned as a novel species based on a <70% ratio of DNA homology with other Gordonia bacteria.
Hydrocarbonoclastic bacteria are a heterogeneous group of prokaryotes which can degrade and utilize hydrocarbon compounds as source of carbon and energy. Despite being present in most of environments around the world, several of these specialized bacteria live in the sea and have been isolated from polluted seawater.

A plastivore is an organism capable of degrading and metabolising plastic. While plastic is normally thought of as non-biodegradable, a variety of bacteria, fungi and insects have been found to degrade it.

Methyl violet 6B is a violet triarylmethane dye from the group of cationic dyes and an essential component of C.I. Basic Violet 1. The compound is sometimes equated with methyl violet in the literature.

Methyl violet 2B (Tetramethylparosanilinium chloride, 4,4′-[(4-imino-2,5-cyclohexadien-1-yliden)methylen]bis(N,N-dimethylaniline)hydrochloride) is a violet triarylmethane dye from the group of cationic dyes and an essential component of C.I. Basic Violet 1 (trivial name methyl violet). Methyl violets are mixtures of tetramethyl (2B), pentamethyl (6B) and hexamethyl (10B) pararosanilins.

Green photocatalysts are photocatalysts derived from environmentally friendly sources. They are synthesized from natural, renewable, and biological resources, such as plant extracts, biomass, or microorganisms, minimizing the use of toxic chemicals and reducing the environmental impact associated with conventional photocatalyst production.



















