Related Research Articles

Adenylate cyclase is an enzyme with systematic name ATP diphosphate-lyase . It catalyzes the following reaction:
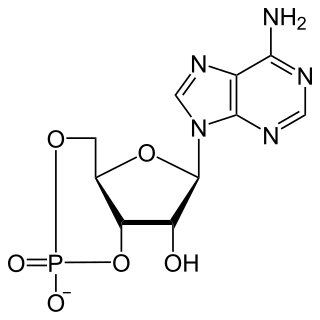
Cyclic adenosine monophosphate is a second messenger, or cellular signal occurring within cells, that is important in many biological processes. cAMP is a derivative of adenosine triphosphate (ATP) and used for intracellular signal transduction in many different organisms, conveying the cAMP-dependent pathway.

G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Ligands can bind either to the extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists, although a spontaneous auto-activation of an empty receptor has also been observed.

G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their activity is regulated by factors that control their ability to bind to and hydrolyze guanosine triphosphate (GTP) to guanosine diphosphate (GDP). When they are bound to GTP, they are 'on', and, when they are bound to GDP, they are 'off'. G proteins belong to the larger group of enzymes called GTPases.
Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.

The sodium–potassium pump is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.

Calcium ions (Ca2+) contribute to the physiology and biochemistry of organisms' cells. They play an important role in signal transduction pathways, where they act as a second messenger, in neurotransmitter release from neurons, in contraction of all muscle cell types, and in fertilization. Many enzymes require calcium ions as a cofactor, including several of the coagulation factors. Extracellular calcium is also important for maintaining the potential difference across excitable cell membranes, as well as proper bone formation.

Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.

CREB-TF is a cellular transcription factor. It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the genes. CREB was first described in 1987 as a cAMP-responsive transcription factor regulating the somatostatin gene.

Cyclic nucleotide–gated ion channels or CNG channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various tissue and cell types, and are significant in sensory transduction as well as cellular development. Their function can be the result of a combination of the binding of cyclic nucleotides and either a depolarization or a hyperpolarization event. Initially discovered in the cells that make up the retina of the eye, CNG channels have been found in many different cell types across both the animal and the plant kingdoms. CNG channels have a very complex structure with various subunits and domains that play a critical role in their function. CNG channels are significant in the function of various sensory pathways including vision and olfaction, as well as in other key cellular functions such as hormone release and chemotaxis. CNG channels have also been found to exist in prokaryotes, including many spirochaeta, though their precise role in bacterial physiology remains unknown.
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.

The Babraham Institute is a life sciences research institution focussing on healthy ageing. The Babraham Institute is based on the Babraham Research Campus, partly occupying a former manor house, but also laboratory and science facility buildings on the campus, surrounded by an extensive parkland estate, just south of Cambridge, England. It is an independent and charitable organization which is involved in biomedical research, including healthy aging and molecular biology. The director is Dr Simon Cook who also leads the Institute's signalling research programme.
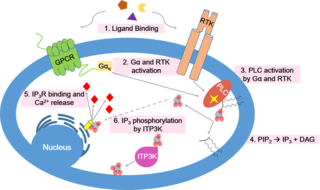
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.

In the nervous system, a synapse is a structure that permits a neuron to pass an electrical or chemical signal to another neuron or to the target effector cell.

Nicotinic acid adenine dinucleotide phosphate (NAADP) is a Ca2+-mobilizing second messenger synthesised in response to extracellular stimuli. Like its mechanistic cousins, IP3 and cyclic adenosine diphosphoribose (Cyclic ADP-ribose), NAADP binds to and opens Ca2+ channels on intracellular organelles, thereby increasing the intracellular Ca2+ concentration which, in turn, modulates sundry cellular processes (see Calcium signalling). Structurally, it is a dinucleotide that only differs from the house-keeping enzyme cofactor, NADP by a hydroxyl group (replacing the nicotinamide amino group) and yet this minor modification converts it into the most potent Ca2+-mobilizing second messenger yet described. NAADP acts across phyla from plants to humans.

2-Aminoethoxydiphenyl borate (2-APB) is a chemical that acts to inhibit both IP3 receptors and TRP channels (although it activates TRPV1, TRPV2, & TRPV3 at higher concentrations). In research it is used to manipulate intracellular release of calcium ions (Ca2+) and modify TRP channel activity, although the lack of specific effects make it less than ideal under some circumstances. Additionally, there is evidence that 2-APB acts directly to inhibit gap junctions made of connexin. Increasing evidence showed that 2-APB is a powerful modifier of store-operated calcium channels (SOC) function, low concentration of 2-APB can enhance SOC while high concentration induces a transient increase followed by complete inhibition.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.
In the field of molecular biology, the cAMP-dependent pathway, also known as the adenylyl cyclase pathway, is a G protein-coupled receptor-triggered signaling cascade used in cell communication.
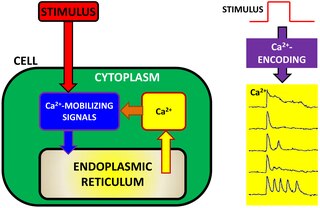
Calcium encoding (also referred to as Ca2+ encoding or calcium information processing) is an intracellular signaling pathway used by many cells to transfer, process and encode external information detected by the cell. In cell physiology, external information is often converted into intracellular calcium dynamics. The concept of calcium encoding explains how Ca2+ ions act as intracellular messengers, relaying information within cells to regulate their activity. Given the ubiquity of Ca2+ ions in cell physiology, Ca2+ encoding has also been suggested as a potential tool to characterize cell physiology in health and disease. The mathematical bases of Ca2+ encoding have been pioneered by work of Joel Keizer and Hans G. Othmer on calcium modeling in the 1990s and more recently they have been revisited by Eshel Ben-Jacob, Herbert Levine and co-workers.

Antony Giuseppe Galione is a British pharmacologist. He is a professor and Wellcome Trust senior investigator in the Department of Pharmacology at the University of Oxford.
References
- 1 2 "Sir Michael Berridge, biochemist behind a paradigm shift in cell science – obituary". The Telegraph . 23 February 2020. Archived from the original on 27 February 2020. Retrieved 27 February 2020.
- 1 2 3 4 "Prof. Dr. Michael J. Berridge" (PDF). Ernst Schering Foundation. 21 November 2017. Archived from the original (PDF) on 3 November 2023. Retrieved 3 November 2023.
- ↑ "Antony Galione". New College, Oxford. Archived from the original on 6 November 2023. Retrieved 6 November 2023.
- ↑ Petersen, Ole H. (2024). "Sir Michael John Berridge. 22 October 1938 — 13 February 2020". Biographical Memoirs of Fellows of the Royal Society. 76.
- 1 2 3 4 5 Berridge, Michael J. (2005). "Unlocking the secrets of cell signaling". Annual Review of Physiology . 67 (1): 1–21. doi: 10.1146/annurev.physiol.67.040103.152647 . PMID 15709950.
- 1 2 "Autobiography of Michael Berridge". Shaw Prize. Archived from the original on 7 November 2023. Retrieved 7 November 2023.
- 1 2 3 4 5 "Sir Michael John Berridge". Academia Europaea. Archived from the original on 8 November 2023. Retrieved 8 November 2023.
- ↑ "In tribute: Sir Michael Berridge FRS". Babraham Institute. 13 February 2020. Archived from the original on 11 November 2023. Retrieved 11 November 2023.
- 1 2 "The Prize in Life Science & Medicine 2005". Shaw Prize. Archived from the original on 11 November 2023. Retrieved 11 November 2023.
- ↑ "Cell Signalling Biology". Biochemical Society. Archived from the original on 19 December 2023. Retrieved 19 December 2023.
- 1 2 Berridge, Mike (2005). "Interview with Mike Berridge". BioEssays . 27 (2): 201–210. doi:10.1002/bies.20186. PMID 15666351 . Retrieved 23 November 2023.
- ↑ Berridge, M. J.; Patel, N. G. (1968). "Insect Salivary Glands: Stimulation of Fluid Secretion by 5-Hydroxytryptamine and Adenosine-3′,5′-monophosphate". Science . 162 (3852): 462–463. doi:10.1126/science.162.3852.462. PMID 4300804. S2CID 45803569 . Retrieved 23 November 2023.
- ↑ Berridge, Michael J.; Prince, William T. (1972). "Transepithelial potential changes during stimulation of isolated salivary glands with 5-hydroxytryptamine and cyclic AMP". Journal of Experimental Biology . 56 (1): 139–153. doi: 10.1242/jeb.56.1.139 . PMID 4339528. Archived from the original on 28 November 2023. Retrieved 28 November 2023.
- ↑ Prince, William T.; Berridge, Michael J.; Rasmussen, Howard (1972). "Role of Calcium and Adenosine-3′:5′-Cyclic Monophosphate in Controlling Fly Salivary Gland Secretion". Proceedings of the National Academy of Sciences of the United States of America . 69 (3): 553–557. doi: 10.1073/pnas.69.3.553 . PMC 426505 . PMID 4335064.
- ↑ Berridge, Michael John. (1981). "Electrophysiological evidence for the existence of separate receptor mechanisms mediating the action of 5-hydroxytryptamine". Molecular and Cellular Endocrinology . 23 (1): 91–104. doi:10.1016/0303-7207(81)90119-2. PMID 6266901. S2CID 38874406 . Retrieved 29 November 2023.
- ↑ Robert H., Michell (1975). "Inositol phospholipids and cell surface receptor function". Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes . 415 (1): 81–147. doi:10.1016/0304-4157(75)90017-9. PMID 164246 . Retrieved 29 November 2023.
- ↑ Berridge, Michael J.; Dawson, Rex M. C.; Downes, C. Peter; Heslop, John P.; Irvine, Robin F. (1983). "Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides". Biochemical Journal . 212 (2): 473–482. doi:10.1042/bj2120473. PMC 1152070 . PMID 6309146.
- ↑ Streb, H.; Irvine, R. F.; Berridge, M. J.; Schulz, I. (1983). "Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate". Nature . 306 (5938): 67–69. Bibcode:1983Natur.306...67S. doi:10.1038/306067a0. PMID 6605482. S2CID 4359904 . Retrieved 19 December 2023.
- ↑ Nishizuka, Yasutomi (1984). "The role of protein kinase C in cell surface signal transduction and tumour promotion". Nature. 308 (5961): 693–698. Bibcode:1984Natur.308..693N. doi:10.1038/308693a0. PMID 6232463. S2CID 4240505.
- ↑ Berridge, Michael J.; Bootman, Martin D.; Roderick, H. Llewelyn (2003). "Calcium signalling: dynamics, homeostasis and remodelling". Nature Reviews Molecular Cell Biology . 4 (7): 517–529. doi:10.1038/nrm1155. PMID 12838335. S2CID 1152297 . Retrieved 19 December 2023.
- ↑ "Michael Berridge". Royal Society. Archived from the original on 17 November 2023. Retrieved 17 November 2023.
- ↑ "Professor Michael J. Berridge". King Faisal Prize. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Professor Michael BERRIDGE". Louis-Jeantet Foundation. October 2017. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Michael J. Berridge". Gairdner Foundation. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Sir Michael Berridge". Academia Europaea. Archived from the original on 17 November 2023. Retrieved 17 November 2023.
- ↑ "1989 Albert Lasker Basic Medical Research Award". Lasker Award. Archived from the original on 2 November 2023. Retrieved 2 November 2023.
- ↑ "Michael J. Berridge". European Molecular Biology Organization. Archived from the original on 17 November 2023. Retrieved 17 November 2023.
- ↑ "BERRIDGE Michael". Royal Academy of Medicine of Belgium (Académie royale de médecine de Belgique). Archived from the original on 29 January 2023. Retrieved 29 January 2023.
- ↑ "Award winners : Royal Medal". Royal Society. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ↑ "Sir Michael J. Berridge". Heineken Prizes. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Michael J. Berridge". Wolf Foundation. 10 December 2018. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Massry Prize Winners ( 1996 – Present )". Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "No. 54993". The London Gazette (1st supplement). 30 December 1997. p. 1.
- ↑ "Gonville and Caius College". Cambridge University Reporter . Vol. CXXIX, no. 6. 4 November 1998. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Sir Michael Berridge FRS FMedSci". Academy of Medical Sciences. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ↑ "Michael J. Berridge". Ernst Schering Foundation. Archived from the original on 14 November 2023. Retrieved 14 November 2023.
- ↑ "Michael J. Berridge". National Academy of Sciences. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ↑ "Michael John Berridge". American Academy of Arts and Sciences. 31 May 2023. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ↑ "Honorary Members". Biochemical Society. Archived from the original on 20 November 2023. Retrieved 20 November 2023.
- ↑ "Sir Michael J. Berridge". American Philosophical Society. Archived from the original on 2 November 2023. Retrieved 2 November 2023.
- ↑ "The Sir Michael Berridge Prize". Babraham Institute. Archived from the original on 11 November 2023. Retrieved 11 November 2023.