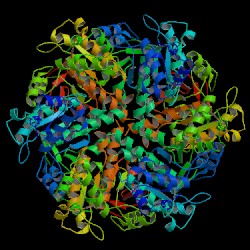
DNA repair protein RAD51 homolog 1 is a protein encoded by the gene RAD51. The enzyme encoded by this gene is a member of the RAD51 protein family which assists in repair of DNA double strand breaks. RAD51 family members are homologous to the bacterial RecA, Archaeal RadA and yeast Rad51. The protein is highly conserved in most eukaryotes, from yeast to humans.
In molecular biology miR-181 microRNA precursor is a small non-coding RNA molecule. MicroRNAs (miRNAs) are transcribed as ~70 nucleotide precursors and subsequently processed by the RNase-III type enzyme Dicer to give a ~22 nucleotide mature product. In this case the mature sequence comes from the 5' arm of the precursor. They target and modulate protein expression by inhibiting translation and / or inducing degradation of target messenger RNAs. This new class of genes has recently been shown to play a central role in malignant transformation. miRNA are downregulated in many tumors and thus appear to function as tumor suppressor genes. The mature products miR-181a, miR-181b, miR-181c or miR-181d are thought to have regulatory roles at posttranscriptional level, through complementarity to target mRNAs. miR-181 which has been predicted or experimentally confirmed in a wide number of vertebrate species as rat, zebrafish, and in the pufferfish.

There are 89 known sequences today in the microRNA 19 (miR-19) family but it will change quickly. They are found in a large number of vertebrate species. The miR-19 microRNA precursor is a small non-coding RNA molecule that regulates gene expression. Within the human and mouse genome there are three copies of this microRNA that are processed from multiple predicted precursor hairpins:
A metastasis suppressor is a protein that acts to slow or prevent metastases from spreading in the body of an organism with cancer. Metastasis is one of the most lethal cancer processes. This process is responsible for about ninety percent of human cancer deaths. Proteins that act to slow or prevent metastases are different from those that act to suppress tumor growth. Genes for about a dozen such proteins are known in humans and other animals.

Epithelial membrane protein 3 (EMP3) is a trans-membrane signaling molecule that is encoded by the myelin-related gene EMP3. EMP3 is a member of the peripheral myelin protein gene family 22-kDa (PMP22), which is mainly responsible for the formation of the sheath of compact myelin. Although the detailed functions and mechanisms of EMP3 still remain unclear, it is suggested that EMP3 is possibly epigenetically linked to certain carcinomas.

microRNA 21 also known as hsa-mir-21 or miRNA21 is a mammalian microRNA that is encoded by the MIR21 gene.
An oncomir is a microRNA (miRNA) that is associated with cancer. MicroRNAs are short RNA molecules about 22 nucleotides in length. Essentially, miRNAs specifically target certain messenger RNAs (mRNAs) to prevent them from coding for a specific protein. The dysregulation of certain microRNAs (oncomirs) has been associated with specific cancer forming (oncogenic) events. Many different oncomirs have been identified in numerous types of human cancers.

In molecular biology, miR-184 microRNA is a short non-coding RNA molecule. MicroRNAs (miRNAs) function as posttranscriptional regulators of expression levels of other genes by several mechanisms. Several targets for miR-184 have been described, including that of mediators of neurological development, apoptosis and it has been suggested that miR-184 plays an essential role in development.

In molecular biology miR-205 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. They are involved in numerous cellular processes, including development, proliferation, and apoptosis. Currently, it is believed that miRNAs elicit their effect by silencing the expression of target genes.

In molecular biology mir-22 microRNA is a short RNA molecule. MicroRNAs are an abundant class of molecules, approximately 22 nucleotides in length, which can post-transcriptionally regulate gene expression by binding to the 3' UTR of mRNAs expressed in a cell.
miR-31 has been characterised as a tumour suppressor miRNA, with its levels varying in breast cancer cells according to the metastatic state of the tumour. From its typical abundance in healthy tissue is a moderate decrease in non-metastatic breast cancer cell lines, and levels are almost completely absent in mouse and human metastatic breast cancer cell lines. Mir-31-5p has also been observed upregulated in Zinc Deficient rats compared to normal in ESCC and in other types of cancers when using this animal model. There has also been observed a strong encapsulation of tumour cells expressing miR-31, as well as a reduced cell survival rate. miR-31's antimetastatic effects therefore make it a potential therapeutic target for breast cancer. However, these two papers were formally retracted by the authors in 2015.

In molecular biology, mir-221 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.

miR-138 is a family of microRNA precursors found in animals, including humans. MicroRNAs are typically transcribed as ~70 nucleotide precursors and subsequently processed by the Dicer enzyme to give a ~22 nucleotide product. The excised region or, mature product, of the miR-138 precursor is the microRNA mir-138.

HOXA11-AS lncRNA is a long non-coding RNA from the antisense strand in the homeobox A. The HOX gene contains four clusters. The sense strand of the HOXA gene codes for proteins. Alternative names for HOXA11-AS lncRNA are: HOXA-AS5, HOXA11S, HOXA11-AS1, HOXA11AS, or NCRNA00076. This gene is 3,885 nucleotides long and resides at chromosome 7 (7p15.2) and is transcribed from an independent gene promoter. Being a lncRNA, it is longer than 200 nucleotides in length, in contrast to regular non-coding RNAs.
In molecular biology, competing endogenous RNAs regulate other RNA transcripts by competing for shared microRNAs (miRNAs). Models for ceRNA regulation describe how changes in the expression of one or multiple miRNA targets alter the number of unbound miRNAs and lead to observable changes in miRNA activity - i.e., the abundance of other miRNA targets. Models of ceRNA regulation differ greatly. Some describe the kinetics of target-miRNA-target interactions, where changes in the expression of one target species sequester one miRNA species and lead to changes in the dysregulation of the other target species. Others attempt to model more realistic cellular scenarios, where multiple RNA targets are affecting multiple miRNAs and where each target pair is co-regulated by multiple miRNA species. Some models focus on mRNA 3' UTRs as targets, and others consider long non-coding RNA targets as well. It's evident that our molecular-biochemical understanding of ceRNA regulation remains incomplete.
In molecular biology mir-25 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. mir-25 levels increase in human heart failure, and treatment with an anti-sense RNA molecule (antagomiR) was recently reported to halt disease progression and improves cardiac function in a mouse heart failure model.

MicroRNA 489 is a miRNA that in humans is encoded by the MIR489 gene.
miR-324-5p is a microRNA that functions in cell growth, apoptosis, cancer, epilepsy, neuronal differentiation, psychiatric conditions, cardiac disease pathology, and more. As a microRNA, it regulates gene expression through targeting mRNAs. Additionally, miR-324-5p is both an intracellular miRNA, meaning it is commonly found within the microenvironment of the cell, and one of several circulating miRNAs found throughout the body. Its presence throughout the body both within and external to cells may contribute to miR-324-5p's wide array of functions and role in numerous disease pathologies – especially cancer – in various organ systems.

MIR22HG, also known as C17orf91, MGC14376, MIRN22, hsa-mir-22, and miR-22 is a human gene that encodes a noncoding RNA (ncRNA).This RNA molecule is not translated into a protein but nonetheless may have important functions.
MicroRNA-125 (miR-125) is a highly conserved microRNA family consisting of miR-125a and miR-125b. MiR-125 can be found throughout diverse species from nematode to humans. MiR-125 family members are involved in cell differentiation, proliferation and apoptosis as a result of targeting messenger RNAs related to these cellular processes. By affecting these cellular processes, miR-125 can cause promotion or suppression of pathological processes including carcinogenesis, muscle abnormalities, neurological disorders and pathologies of the immune system. Moreover, miR-125 also plays an important role in normal immune functions and was described to affect development and function of immune cells as well as playing role in immunological host defense in response to bacterial and viral infections.













