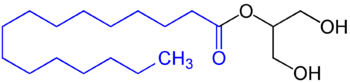
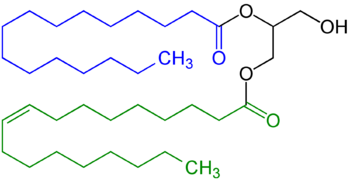
A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

Vegetable oils, or vegetable fats, are oils extracted from seeds or from other parts of fruits. Like animal fats, vegetable fats are mixtures of triglycerides. Soybean oil, grape seed oil, and cocoa butter are examples of seed oils, or fats from seeds. Olive oil, palm oil, and rice bran oil are examples of fats from other parts of fruits. In common usage, vegetable oil may refer exclusively to vegetable fats which are liquid at room temperature. Vegetable oils are usually edible.

Margarine is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was originally named oleomargarine from Latin for oleum and Greek margarite. The name was later shortened to margarine.
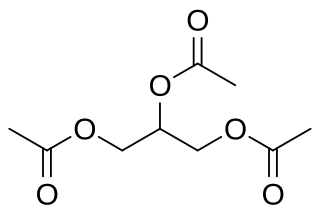
Glycerides, also known as acylglycerols, are esters formed from glycerol and fatty acids, and are generally very hydrophobic.

Lecithin is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues which are amphiphilic – they attract both water and fatty substances, and are used for smoothing food textures, emulsifying, homogenizing liquid mixtures, and repelling sticking materials.
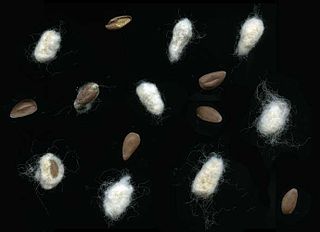
Cottonseed oil is cooking oil from the seeds of cotton plants of various species, mainly Gossypium hirsutum and Gossypium herbaceum, that are grown for cotton fiber, animal feed, and oil.

Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish due to the presence of impurities. In chemical terms, oleic acid is classified as a monounsaturated omega-9 fatty acid, abbreviated with a lipid number of 18:1 cis-9, and a main product of Δ9-desaturase. It has the formula CH3−(CH2)7−CH=CH−(CH2)7−COOH. The name derives from the Latin word oleum, which means oil. It is the most common fatty acid in nature. The salts and esters of oleic acid are called oleates. It is a common component of oils, and thus occurs in many types of food, as well as in soap.

DATEM is an emulsifier primarily used in baking to strengthen the gluten network in dough. It is added to crusty breads, such as rye, to impart a springy, chewy texture. It is also used in the production of biscuits, coffee whiteners, salsa con queso, ice cream, and salad dressings.

Saponification value or saponification number represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value are more suitable for soap making.

Soybean oil is a vegetable oil extracted from the seeds of the soybean. It is one of the most widely consumed cooking oils and the second most consumed vegetable oil. As a drying oil, processed soybean oil is also used as a base for printing inks and oil paints.

Monoglycerides are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol.

Polyglycerol polyricinoleate (PGPR), E476, is an emulsifier made from glycerol and fatty acids. In chocolate, compound chocolate and similar coatings, PGPR is mainly used with another substance like lecithin to reduce viscosity. It is used at low levels, and works by decreasing the friction between the solid particles in molten chocolate, reducing the yield stress so that it flows more easily, approaching the behaviour of a Newtonian fluid. It can also be used as an emulsifier in spreads and in salad dressings, or to improve the texture of baked goods. It is made up of a short chain of glycerol molecules connected by ether bonds, with ricinoleic acid side chains connected by ester bonds.

A dough conditioner, flour treatment agent, improving agent or bread improver is any ingredient or chemical added to bread dough to strengthen its texture or otherwise improve it in some way. Dough conditioners may include enzymes, yeast nutrients, mineral salts, oxidants and reductants, bleaching agents and emulsifiers. They are food additives combined with flour to improve baking functionality. Flour treatment agents are used to increase the speed of dough rising and to improve the strength and workability of the dough.

In organic chemistry glycerolysis refers to any process in which chemical bonds are broken via a reaction with glycerol. The term refers almost exclusively to the transesterification reaction of glycerol with triglycerides (fats/oils) to form mixtures of monoglycerides and diglycerides. These find a variety of uses; as food emulsifiers, 'low fat' cooking oils and surfactants.
Glycerol monostearate, commonly known as GMS, is a monoglyceride commonly used as an emulsifier in foods. It takes the form of a white, odorless, and sweet-tasting flaky powder that is hygroscopic. Chemically it is the glycerol ester of stearic acid. It is also used as hydration powder in exercise formulas

A diglyceride, or diacylglycerol (DAG), is a glyceride consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. Two possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols. Diglycerides are natural components of food fats, though minor in comparison to triglycerides. DAGs can act as surfactants and are commonly used as emulsifiers in processed foods. DAG-enriched oil has been investigated extensively as a fat substitute due to its ability to suppress the accumulation of body fat; with total annual sales of approximately USD 200 million in Japan since its introduction in the late 1990s till 2009.

Cooking oil is a plant or animal liquid fat used in frying, baking, and other types of cooking. Oil allows higher cooking temperatures than water, making cooking faster and more flavorful, while likewise distributing heat, reducing burning and uneven cooking. It sometimes imparts its own flavor. Cooking oil is also used in food preparation and flavoring not involving heat, such as salad dressings and bread dips.

Trans fat, also called trans-unsaturated fatty acids, or trans fatty acids, is a type of unsaturated fat that occurs in foods. Trace concentrations of trans fats occur naturally, but large amounts are found in some processed foods. Since consumption of trans fats is unhealthy, artificial trans fats are highly regulated or banned in many nations. However, they are still widely consumed in developing nations, resulting in hundreds of thousands of deaths each year. The World Health Organization (WHO) had set a goal to make the world free from industrially produced trans fat by the end of 2023. The goal was not met, and the WHO announced another goal "for accelerated action till 2025 to complete this effort" along with associated support on 1 February 2024.

Fat hydrogenation is the process of combining unsaturated fat with hydrogen in order to partially or completely convert it into saturated fat. Typically this hydrogenation is done with liquid vegetable oils resulting in solid or semi-solid fats.