| |||
| Names | |||
|---|---|---|---|
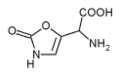
| IUPAC name 2-Amino-2-(2-oxo-3H-1,3-oxazol-5-yl)acetic acid | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.017.141 | ||
| EC Number |
| ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C5H6N2O4 | |||
| Molar mass | 158.113 g·mol−1 | ||
| Appearance | Crystalline solid | ||
| Melting point | 190 °C (374 °F; 463 K) (decomposes) [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Muscazone is a toxic chemical compound. It is an amino acid found in European fly agaric mushrooms. [1]
Consumption causes visual damage, mental confusion, and memory loss. [2]