A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

Influenza vaccines, also known as flu shots, are vaccines that protect against infection by influenza viruses. New versions of the vaccines are developed twice a year, as the influenza virus rapidly changes. While their effectiveness varies from year to year, most provide modest to high protection against influenza. The United States Centers for Disease Control and Prevention (CDC) estimates that vaccination against influenza reduces sickness, medical visits, hospitalizations, and deaths. Immunized workers who do catch the flu return to work half a day sooner on average. Vaccine effectiveness in those over 65 years old remains uncertain due to a lack of high-quality research.
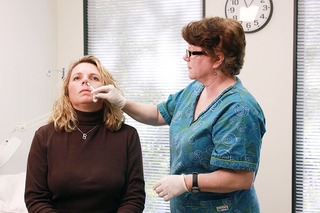
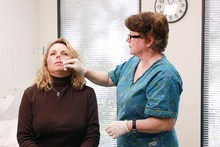
Live attenuated influenza vaccine (LAIV) is a type of influenza vaccine in the form of a nasal spray that is recommended for the prevention of influenza.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.

Original antigenic sin, also known as antigenic imprinting, the Hoskins effect, or immunological imprinting, is the propensity of the immune system to preferentially use immunological memory based on a previous infection when a second slightly different version of that foreign pathogen is encountered. This leaves the immune system "trapped" by the first response it has made to each antigen, and unable to mount potentially more effective responses during subsequent infections. Antibodies or T-cells induced during infections with the first variant of the pathogen are subject to repertoire freeze, a form of original antigenic sin.

MedImmune, LLC was a wholly owned subsidiary of AstraZeneca before February 14, 2019, when it was announced that the MedImmune name and branding would be discontinued in favor of AstraZeneca.

Influenza B virus is the only species in the genus Betainfluenzavirus in the virus family Orthomyxoviridae.
An attenuated vaccine is a vaccine created by reducing the virulence of a pathogen, but still keeping it viable. Attenuation takes an infectious agent and alters it so that it becomes harmless or less virulent. These vaccines contrast to those produced by "killing" the pathogen.

Influenza, commonly known as "the flu", is an infectious disease caused by influenza viruses. Symptoms range from mild to severe and often include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. These symptoms begin from one to four days after exposure to the virus and last for about 2–8 days. Diarrhea and vomiting can occur, particularly in children. Influenza may progress to pneumonia, which can be caused by the virus or by a subsequent bacterial infection. Other complications of infection include acute respiratory distress syndrome, meningitis, encephalitis, and worsening of pre-existing health problems such as asthma and cardiovascular disease.
Immune stimulating complexes (ISCOMs) are spherical open cage-like structures (typically 40 nm in diameter) that are spontaneously formed when mixing together cholesterol, phospholipids and Quillaja saponins under a specific stoichiometry. The complex displays immune stimulating properties and is thus mainly used as a vaccine adjuvant in order to induce a stronger immune response and longer protection.
A subunit vaccine is a vaccine that contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. Subunit vaccine can be made from dissembled viral particles in cell culture or recombinant DNA expression, in which case it is a recombinant subunit vaccine.
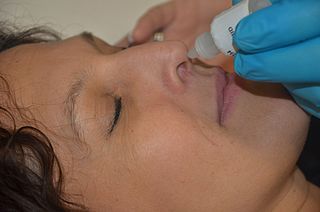
Nasal administration, popularly known as snorting, is a route of administration in which drugs are insufflated through the nose. It can be a form of either topical administration or systemic administration, as the drugs thus locally delivered can go on to have either purely local or systemic effects. Nasal sprays are locally acting drugs such as decongestants for cold and allergy treatment, whose systemic effects are usually minimal. Examples of systemically active drugs available as nasal sprays are migraine drugs, rescue medications for overdose and seizure emergencies, nicotine replacement, and hormone treatments.
Nasal- or nasopharynx- associated lymphoid tissue (NALT) represents immune system of nasal mucosa and is a part of mucosa-associated lymphoid tissue (MALT) in mammals. It protects body from airborne viruses and other infectious agents. In humans, NALT is considered analogous to Waldeyer's ring.
Vaccine shedding is a form of viral shedding which can occur following a viral infection caused by an attenuated vaccine. Illness in others resulting from transmission through this type of viral shedding is rare. Most vaccines are not attenuated vaccines, and therefore cannot cause vaccine-induced viral shedding.

An mRNAvaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules of antigen-encoding mRNA into immune cells, which use the designed mRNA as a blueprint to build foreign protein that would normally be produced by a pathogen or by a cancer cell. These protein molecules stimulate an adaptive immune response that teaches the body to identify and destroy the corresponding pathogen or cancer cells. The mRNA is delivered by a co-formulation of the RNA encapsulated in lipid nanoparticles that protect the RNA strands and help their absorption into the cells.

Vaxart, Inc. is an American biotechnology company focused on the discovery, development, and commercialization of oral recombinant vaccines administered using temperature-stable tablets that can be stored and shipped without refrigeration, eliminating the need for needle injection. Its development programs for oral vaccine delivery include prophylactic, enteric-coated tablet vaccines for inhibiting norovirus, seasonal influenza, respiratory syncytial virus, and human papillomavirus. It was founded in 2004 by Sean Tucker.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.

Daniela M. Ferreira is a Brazilian British immunologist. She is a specialist in bacterial infection, respiratory co-infection, mucosal immunology and vaccine responses. She is currently Professor of Respiratory Infection and Vaccinology at the Oxford Vaccine Group in the Department of Paediatrics at the University of Oxford and the Director of the Liverpool Vaccine Group at the Liverpool School of Tropical Medicine. She leads a team of scientists studying protective immune responses against pneumococcus and other respiratory pathogens such as SARS-CoV2. Her team has established a novel method of inducing pneumococcal carriage in human volunteers. They use this model to:
Type A influenza vaccine is for the prevention of infection of influenza A virus and also the influenza-related complications. Different monovalent type A influenza vaccines have been developed for different subtypes of influenza A virus including H1N1 and H5N1. Both intramuscular injection or intranasal spray are available on market. Unlike the seasonal influenza vaccines which are used annually, they are usually used during the outbreak of certain strand of subtypes of influenza A. Common adverse effects includes injection site reaction and local tenderness. Incidences of headache and myalgia were also reported with H1N1 whereas cases of fever has also been demonstrated with H5N1 vaccines. It is stated that immunosuppressant therapies would reduce the therapeutic effects of vaccines and that people with egg allergy should go for the egg-free preparations.
Live recombinant vaccines are biological preparations that improve immunity through the use of live bacteria or viruses that are genetically modified. These live pathogens are biologically engineered to express exogenous antigens in the cytoplasm of target cells, triggering immune responses as a result. This form of vaccine combines the beneficial features of attenuated and recombinant vaccines, providing the preparation with attenuated vaccines’ long-lasting immunity and recombinant vaccines’ genetically engineered precision and safety.