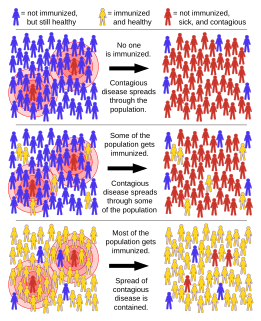
Herd immunity is a form of indirect protection that applies only to contagious diseases. It occurs when a sufficient percentage of a population has become immune to an infection, whether through previous infections or vaccination, thereby reducing the likelihood of infection for individuals who lack immunity.

Kennel cough, also known as canine infectious respiratory disease, formally canine infectious tracheobronchitis, is an upper respiratory infection affecting dogs. There are multiple causative agents, the most common being the bacterium Bordetella bronchiseptica, followed by canine parainfluenza virus, and to a lesser extent canine coronavirus. It is highly contagious; however, adult dogs may display immunity to reinfection even under constant exposure. Kennel cough is so named because the infection can spread quickly among dogs in the close quarters of a kennel or animal shelter.
Marek's disease is a highly contagious viral neoplastic disease in chickens. It is named after József Marek, a Hungarian veterinarian who described it in 1907. Marek's disease is caused by an alphaherpesvirus known as "Marek's disease virus" (MDV) or Gallid alphaherpesvirus 2 (GaHV-2). The disease is characterized by the presence of T cell lymphoma as well as infiltration of nerves and organs by lymphocytes. Viruses related to MDV appear to be benign and can be used as vaccine strains to prevent Marek's disease. For example, the related herpesvirus found in turkeys (HVT), causes no apparent disease in the birds, and continues to be used as a vaccine strain for prevention of Marek's disease.
Canid alphaherpesvirus 1 (CaHV-1), formerly Canine herpesvirus (CHV), is a virus of the family Herpesviridae which most importantly causes a fatal hemorrhagic disease in puppies less than two to three weeks old. It is known to exist in the United States, Canada, Australia, Japan, England and Germany. CHV was first recognized in the mid-1960s from a fatal disease in puppies.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections. The occurrence of latent infections caused by these viruses could be linked to the genome's abundance in inversions which facilitate viral genome integration.
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.
Aujeszky's disease, usually called pseudorabies in the United States, is a viral disease in swine that has been endemic in most parts of the world. It is caused by Suid herpesvirus 1 (SuHV-1). Aujeszky's disease is considered to be the most economically important viral disease of swine in areas where classical swine fever has been eradicated. Other mammals, such as cattle, sheep, goats, cats, dogs, and raccoons, are also susceptible. The disease is usually fatal in these animal species.
Equid alphaherpesvirus 4, formerly Equine herpesvirus 4 (EHV-4) is a virus of the family Herpesviridae that cause rhinopneumonitis in horses. It is the most important viral cause of respiratory infection in foals. Like other herpes viruses, EHV-4 causes a lifelong latent infection in affected animals. These horses are usually the source for new infection for foals over two months old, weanlings, and yearlings. Symptoms include fever, loss of appetite, and discharge from the nose. Most infected animals recover in one to three weeks, but death can occur in environments with overcrowding and other stress factors. There are several vaccines available.
Equid alphaherpesvirus 1, formerly Equine herpesvirus 1 (EHV-1), is a virus of the family Herpesviridae that causes abortion, respiratory disease and occasionally neonatal mortality in horses. Initial spread of EHV-1 by a newly introduced horse through direct and indirect contact can lead to abortion and perinatal infection in up to 70 percent of a previously unexposed herd. Abortion usually occurs in the last four months of gestation, two to four weeks after infection of the mare. Perinatal infection can lead to pneumonia and death. Encephalitis can occur in affected animals, leading to ataxia, paralysis, and death. There is a vaccine available, however its efficacy is questionable. The virus varies in severity from sub-clinical to very severe. Most horses have been infected with EHV-1, but the virus can become latent and persist without ever causing signs of infection. In 2006, an outbreak of EHV-1 among stables in Florida resulted in the institution of quarantine measures. The outbreak was determined to have originated in horses imported from Europe via New York, before being shipped to Florida.

Feline viral rhinotracheitis (FVR) is an upper respiratory or pulmonary infection of cats caused by Felid alphaherpesvirus 1 (FeHV-1), of the family Herpesviridae. It is also commonly referred to as feline influenza, feline coryza, and feline pneumonia but, as these terms describe other very distinct collections of respiratory symptoms, they are misnomers for the condition. Viral respiratory diseases in cats can be serious, especially in catteries and kennels. Causing one-half of the respiratory diseases in cats, FVR is the most important of these diseases and is found worldwide. The other important cause of feline respiratory disease is feline calicivirus.

Feline calicivirus (FCV) is a virus of the family Caliciviridae that causes disease in cats. It is one of the two important viral causes of respiratory infection in cats, the other being Felid alphaherpesvirus 1. FCV can be isolated from about 50% of cats with upper respiratory infections. Cheetahs are the other species of the family Felidae known to become infected naturally.

Bovine malignant catarrhal fever (BMCF) is a fatal lymphoproliferative disease caused by a group of ruminant gamma herpes viruses including Alcelaphine gammaherpesvirus 1 (AlHV-1) and Ovine gammaherpesvirus 2 (OvHV-2) These viruses cause unapparent infection in their reservoir hosts, but are usually fatal in cattle and other ungulates such as deer, antelope, and buffalo. In Southern Africa the disease is known as snotsiekte, from the Afrikaans.
Bovine alphaherpesvirus 5 (BoHV-5) is a virus species of the genus Varicellovirus and subfamily Alphaherpesvirinae. It causes meningoencephalitis and respiratory disease in cattle and sheep. As with all herpes viruses latent infection can occur, with recrudescence at times of stressed and/or immunosuppression. Sites of latency include the CNS and mucosae of the nose and trachea. The disease has been documented in South America, the United States, Australia, Germany and Hungary. Caused by: BHV-5 – Bovine Encephalitis Virus – Bovine Encephalitis Herpesvirus

Bovine viral diarrhea (BVD), bovine viral diarrhoeaor mucosal disease, and previously referred to as bovine virus diarrhea (BVD), is an economically significant disease of cattle that is found in the majority of countries throughout the world. Worldwide reviews of the economically assessed production losses and intervention programs incurred by BVD infection have been published. The causative agent, bovine viral diarrhea virus (BVDV), is a member of the genus Pestivirus of the family Flaviviridae.
Varicellovirus (var′i-sel′ō-vi′rŭs) is a genus of viruses belonging to subfamily Alphaherpesvirinae, a member of family Herpesviridae. Humans and other mammals serve as natural hosts. There are 19 species in this genus. Diseases associated with this genus include: HHV-3—chickenpox (varicella) and shingles; BoHV-1—infectious bovine rhinotracheitis/infectious pustular vulvovaginitis (IPV); SuHV-1 —Aujesky's disease characterized by central nervous system signs, high mortality rates in young animals, and respiratory illness in older pigs.
Infectious bovine keratoconjunctivitis (IBK), also known as pinkeye, New Forest eye or blight, is a veterinary infection of cattle caused by Moraxella bovis, a Gram-negative, β-haemolytic, aerobic, rod-shaped bacterium. It is spread by direct contact or by flies serving as vectors. It is the most common ocular disease of cattle. IBK is similar to human pink eye and causes severe infection of the conjunctiva, edema, corneal opacity, and ulceration. This disease is highly contagious and occurs worldwide. Younger animals are more susceptible, but recovery with minimal damage is usual, if they are treated early.
Bovine gammaherpesvirus 4 (BoHV-4) is a member of the Herpesviridae family. It is part of the subfamily Gammaherpesvirinae and genus Rhadinovirus. Infection is normally sub-clinical but can cause reproductive disease in cattle such as endometritis, vulvovaginitis and mastitis. Transmission is both vertical and horizontal. It can also be indirectly spread by fomites. Distribution is worldwide and the virus infects a range of ruminants, including bison, buffalo, sheep and goats.
Pacheco's disease is a highly infectious and acute bird disease caused by a species of herpesvirus, Psittacid alphaherpesvirus 1 (PsHV-1). All psittacine species are susceptible to Pacheco's disease, mainly those in zoological collections and aviaries in any geographic regions. Specifically, Pacheco's disease has a high occurrence rate in Amazon parrots, followed by African grey parrots, parrots, macaws, cockatoos and conures. Due to a very high mortality rate within these susceptible species, concerns are brought to companion bird markets and breeders.
Bovine respiratory disease (BRD) is the most common and costly disease affecting beef cattle in the world. It is a complex, bacterial or viral infection that causes pneumonia in calves which can be fatal. The infection is usually a sum of three codependent factors: stress, an underlying viral infection, and a new bacterial infection. The diagnosis of the disease is complex since there are multiple possible causes.
Caprine alphaherpesvirus 1 (CpHV-1) is a species of virus known to infect goats worldwide. It has been shown to produce systemic and respiratory symptoms in kids and to cause abortions in adult goats.







