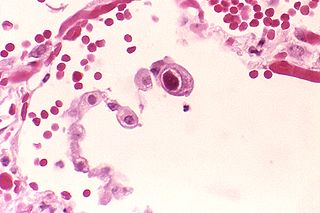
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia, and congenital CMV in infants can lead to deafness and ambulatory problems.

Roseola, also known as sixth disease, is an infectious disease caused by certain types of human herpes viruses. Most infections occur before the age of three. Symptoms vary from absent to the classic presentation of a fever of rapid onset followed by a rash. The fever generally lasts for three to five days, while the rash is generally pink and lasts for less than three days. Complications may include febrile seizures, with serious complications being rare.
Roseolovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. There are currently six species in this genus. Diseases associated with this genus include: HHV-6: sixth disease ; HHV-7: symptoms analog to the 'sixth disease'.
Rhadinovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Gammaherpesvirinae. Humans and other mammals serve as natural hosts. There are 12 species in this genus. Diseases associated with this genus include: Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease, caused by Human gammaherpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV). The term rhadino comes from the Latin fragile, referring to the tendency of the viral genome to break apart when it is isolated.
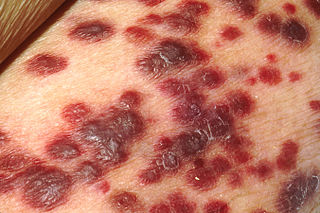
Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.

Alphaherpesvirinae is a subfamily of viruses in the family Herpesviridae, primarily distinguished by reproducing more quickly than other subfamilies in the Herpesviridae. In animal virology the most important herpesviruses belong to the Alphaherpesvirinae. Pseudorabies virus is the causative agent of Aujeszky's disease in pigs and Bovine herpesvirus 1 is the causative agent of bovine infectious rhinotracheitis and pustular vulvovaginitis. Mammals serve as natural hosts. There are currently 45 species in this subfamily, divided among 5 genera with one species unassigned to a genus. Diseases associated with this subfamily include: HHV-1 and HHV-2: skin vesicles or mucosal ulcers, rarely encephalitis and meningitis, HHV-3: chickenpox (varicella) and shingles, GaHV-2: Marek's disease.
Virus latency is the ability of a pathogenic virus to lie dormant within a cell, denoted as the lysogenic part of the viral life cycle. A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny without the host becoming reinfected by new outside virus, and stays within the host indefinitely.

Human herpesvirus 6 (HHV-6) is the common collective name for human betaherpesvirus 6A (HHV-6A) and human betaherpesvirus 6B (HHV-6B). These closely related viruses are two of the nine known herpesviruses that have humans as their primary host.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Duck plague is a worldwide disease caused by Anatid alphaherpesvirus 1 (AnHV-1) of the family Herpesviridae that causes acute disease with high mortality rates in flocks of ducks, geese, and swans. It is spread both vertically and horizontally—through contaminated water and direct contact. Migratory waterfowl are a major factor in the spread of this disease as they are often asymptomatic carriers of disease. The incubation period is three to seven days. Birds as young as one week old can be infected. DEV is not zoonotic.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.

Human betaherpesvirus 5, also called human cytomegalovirus (HCMV), is species of virus in the genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals. CMV is a double-stranded DNA virus.
The latency-associated nuclear antigen (LANA-1) or latent nuclear antigen is a Kaposi's sarcoma-associated herpesvirus (KSHV) latent protein initially found by Moore and colleagues as a speckled nuclear antigen present in primary effusion lymphoma cells that reacts with antibodies from patients with KS. It is the most immunodominant KSHV protein identified by Western-blotting as 222–234 kDa double bands migrate slower than the predicted molecular weight. LANA has been suspected of playing a crucial role in modulating viral and cellular gene expression. It is commonly used as an antigen in blood tests to detect antibodies in persons that have been exposed to KSHV.

The Herpesvirales is an order of dsDNA viruses with animal hosts, characterised by a common morphology consisting of an icosahedral capsid enclosed in a glycoprotein-containing lipid envelope. Common infections in humans caused by members of this order include cold sores, genital herpes, chickenpox, shingles, and glandular fever. Herpesvirales is the sole order in the class Herviviricetes, which is the sole class in the phylum Peploviricota.
Human betaherpesvirus 7 (HHV-7) is one of nine known members of the Herpesviridae family that infects humans. HHV-7 is a member of Betaherpesvirinae, a subfamily of the Herpesviridae that also includes HHV-6 and Cytomegalovirus. HHV-7 often acts together with HHV-6, and the viruses together are sometimes referred to by their genus, Roseolovirus. HHV-7 was first isolated in 1990 from CD4+ T cells taken from peripheral blood lymphocytes.
Muromegalovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Rodents serve as natural hosts. There are three species in this genus. Diseases associated with this genus include: infected peritoneal macrophages, dendritic cells (DC) and hepatocytes, inducing significant pathology in both the spleen and the liver. Murid viruses Murid betaherpesvirus 1 (MuHV-1) and Murid betaherpesvirus 2 (MuHV-2), previously defined as mouse cytomegalovirus (MCMV) and rat cytomegalovirus (RCMV), belong to this genus.
Proboscivirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Elephants serve as natural hosts. EEHV1 is apathogenic for African elephants but causes fatal haemorrhagic disease in Asian elephants. The name "Proboscivirus" comes from the Greek word προβοσκίς or "proboscis" meaning "the elephant trunk," for which the virus accordingly uses as its means of contraction and transmission to enter the elephant's body.
Human herpes viruses, also known as HHVs, are part of a family of DNA viruses that cause several diseases in humans. One of the most notable functions of this virus family is their ability to enter a latent phase and lay dormant within animals for extended periods of time. The mechanism that controls this is very complex because expression of viral proteins during latency is decreased a great deal, meaning that the virus must have transcription of its genes repressed. There are many factors and mechanisms that control this process and epigenetics is one way this is accomplished. Epigenetics refers to persistent changes in expression patterns that are not caused by changes to the DNA sequence. This happens through mechanisms such as methylation and acetylation of histones, DNA methylation, and non-coding RNAs (ncRNA). Altering the acetylation of histones creates changes in expression by changing the binding affinity of histones to DNA, making it harder or easier for transcription machinery to access the DNA. Methyl and acetyl groups can also act as binding sites for transcription factors and enzymes that further modify histones or alter the DNA itself.
Human betaherpesvirus 6A (HHV-6A) is a species of virus in the genus Roseolovirus, subfamily Betaherpesvirinae, family Herpesviridae, and order Herpesvirales.
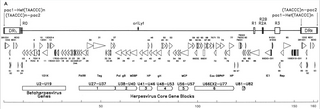
Human betaherpesvirus 6B (HHV-6B) is a species of virus in the genus Roseolovirus, subfamily Betaherpesvirinae, family Herpesviridae, and order Herpesvirales.










