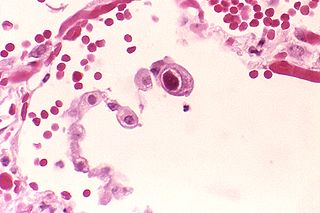
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia, and congenital CMV in infants can lead to deafness and ambulatory problems.

The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus. Epstein–Barr virus (EBV) is the first identified oncogenic virus, which establishes permanent infection in humans. EBV causes infectious mononucleosis and is also tightly linked to many malignant diseases. Various vaccine formulations underwent testing in different animals or in humans. However, none of them were able to prevent EBV infection and no vaccine has been approved to date.

Varicella zoster virus (VZV), also known as human herpesvirus 3 or Human alphaherpesvirus 3 (taxonomically), is one of nine known herpes viruses that can infect humans. It causes chickenpox (varicella) commonly affecting children and young adults, and shingles in adults but rarely in children. As a late complication of VZV infection, Ramsay Hunt syndrome type 2 may develop in rare cases. VZV infections are species-specific to humans. The virus can survive in external environments for a few hours.

Roseola, also known as sixth disease, is an infectious disease caused by certain types of human herpes viruses. Most infections occur before the age of three. Symptoms vary from absent to the classic presentation of a fever of rapid onset followed by a rash. The fever generally lasts for three to five days, while the rash is generally pink and lasts for less than three days. Complications may include febrile seizures, with serious complications being rare.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.

In molecular biology, CD4 is a glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). CD4 is found on the surface of immune cells such as helper T cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984. In humans, the CD4 protein is encoded by the CD4 gene.
Virus latency is the ability of a pathogenic virus to lie dormant within a cell, denoted as the lysogenic part of the viral life cycle. A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny without the host becoming reinfected by new outside virus, and stays within the host indefinitely.

Human herpesvirus 6 (HHV-6) is the common collective name for human betaherpesvirus 6A (HHV-6A) and human betaherpesvirus 6B (HHV-6B). These closely related viruses are two of the nine known herpesviruses that have humans as their primary host.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Herpes simplex virus1 and 2, also known by their taxonomic names Human alphaherpesvirus 1 and Human alphaherpesvirus 2, are two members of the human Herpesviridae family, a set of viruses that produce viral infections in the majority of humans. Both HSV-1 and HSV-2 are very common and contagious. They can be spread when an infected person begins shedding the virus.
Simian foamy virus (SFV) is a species of the genus Spumavirus that belongs to the family of Retroviridae. It has been identified in a wide variety of primates, including prosimians, New World and Old World monkeys, as well as apes, and each species has been shown to harbor a unique (species-specific) strain of SFV, including African green monkeys, baboons, macaques, and chimpanzees. As it is related to the more well-known retrovirus human immunodeficiency virus (HIV), its discovery in primates has led to some speculation that HIV may have been spread to the human species in Africa through contact with blood from apes, monkeys, and other primates, most likely through bushmeat-hunting practices.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.

Betaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Mammals serve as natural hosts. There are 26 species in this subfamily, divided among 5 genera. Diseases associated with this subfamily include: human cytomegalovirus (HHV-5): congenital CMV infection; HHV-6: 'sixth disease' ; HHV-7: symptoms analogous to the 'sixth disease'.

B-virus, Herpesvirus simiae, or Herpes virus B is the Simplexvirus infecting macaque monkeys. B virus is very similar to HSV-1, and as such, this neurotropic virus is not found in the blood.

Human betaherpesvirus 5, also called human cytomegalovirus (HCMV,HHV-5), is a species of virus in the genus Cytomegalovirus, which in turn is a member of the viral family known as Herpesviridae or herpesviruses. It is also commonly called CMV. Within Herpesviridae, HCMV belongs to the Betaherpesvirinae subfamily, which also includes cytomegaloviruses from other mammals. CMV is a double-stranded DNA virus.
The latency-associated nuclear antigen (LANA-1) or latent nuclear antigen is a Kaposi's sarcoma-associated herpesvirus (KSHV) latent protein initially found by Moore and colleagues as a speckled nuclear antigen present in primary effusion lymphoma cells that reacts with antibodies from patients with KS. It is the most immunodominant KSHV protein identified by Western-blotting as 222–234 kDa double bands migrate slower than the predicted molecular weight. LANA has been suspected of playing a crucial role in modulating viral and cellular gene expression. It is commonly used as an antigen in blood tests to detect antibodies in persons that have been exposed to KSHV.
Anthony (Tony) Charles Minson, PhD, FMedSci is a British virologist known for his work on the biology of herpesviruses, and a university administrator. He was the Senior Pro-Vice-Chancellor of the University of Cambridge from 2003 to 2009. He is an emeritus professor of virology at the university's Department of Pathology and an emeritus fellow of Wolfson College.
Elephant endotheliotropic herpesviruses (EEHV) or Elephantid betaherpesvirus 1 (ElHV-1) is a type of herpesvirus, which can cause a highly fatal hemorrhagic disease when transmitted to young Asian elephants. In African elephants, related forms of these viruses, which have been identified in wild populations, are generally benign, occasionally surfacing to cause small growths or lesions. However, some types of EEHV can cause a highly fatal disease in Asian elephants, which kills up to 80% of severely affected individuals. The disease can be treated with the rapid application of antiviral drugs, but this has only been effective in around a third of cases.
Human betaherpesvirus 6A (HHV-6A) is a species of virus in the genus Roseolovirus, subfamily Betaherpesvirinae, family Herpesviridae, and order Herpesvirales.
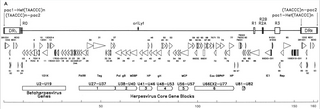
Human betaherpesvirus 6B (HHV-6B) is a species of virus in the genus Roseolovirus, subfamily Betaherpesvirinae, family Herpesviridae, and order Herpesvirales.










