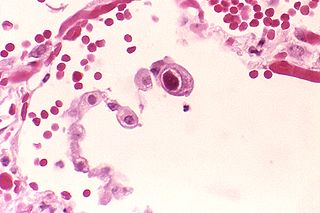
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia. In the medical literature, most mentions of CMV without further specification refer implicitly to human CMV. Human CMV is the most studied of all cytomegaloviruses.

The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus.
Rhadinovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Gammaherpesvirinae. Humans and other mammals serve as natural hosts. There are 12 species in this genus. Diseases associated with this genus include: Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease, caused by Human gammaherpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV). The term rhadino comes from the Latin fragile, referring to the tendency of the viral genome to break apart when it is isolated.

Virus latency is the ability of a pathogenic virus to lie dormant within a cell, denoted as the lysogenic part of the viral life cycle. A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny without the host becoming reinfected by new outside virus, and stays within the host indefinitely.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.

B-virus, Herpesvirus simiae, or Herpes virus B is the Simplexvirus infecting macaque monkeys. B virus is very similar to HSV-1, and as such, this neurotropic virus is not found in the blood.

Lymphocryptovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Gammaherpesvirinae. This genus includes the human-infecting Human gammaherpesvirus 4, as well as viruses that infect both Old World monkeys and New World monkeys. Other names for the Lymphocryptovirus genus include Lymphocryptoviridae and gamma-1 herpesviruses. There are nine species in this genus. Diseases associated with this genus include: mononucleosis, Burkitt's lymphoma, and nasopharyngeal carcinoma.
There are several forms of Epstein–Barr virus (EBV) infection. These include asymptomatic infections, the primary infection, infectious mononucleosis, and the progression of asymptomatic or primary infections to: 1) any one of various Epstein–Barr virus-associated lymphoproliferative diseases such as chronic active EBV infection, EBV+ hemophagocytic lymphohistiocytosis, Burkitt's lymphoma, and Epstein–Barr virus positive diffuse large B-cell lymphoma, not otherwise specified); 2) non-lymphoid cancers such as Epstein–Barr virus associated gastric cancer, soft tissue sarcomas, leiomyosarcoma, and nasopharyngeal cancers; and 3) Epstein–Barr virus-associated non-lymphoproliferative diseases such as some cases of the immune disorders of multiple sclerosis and systemic lupus erythematosis and the childhood disorders of Alice in Wonderland Syndrome and acute cerebellar ataxia.
Epstein–Barr virus latent membrane protein 1 (LMP1) is an Epstein–Barr virus (EBV) protein that regulates its own expression and the expression of human genes. It has a molecular weight of approximately 63 kDa, and its expression induces many of the changes associated with EBV infections and activation of primary B cells. LMP1 is the best-documented oncoprotein of the EBV latent gene products, as it is expressed in most EBV-related human cancers such as the various malignant Epstein-Barr virus-associated lymphoproliferative diseases.

The Epstein–Barr virus–encoded small RNAs (EBERs) are small non-coding RNAs localized in the nucleus of human cells infected with Epstein–Barr virus (EBV). First discovered in 1981, EBERs are the most abundant RNAs present in infected cells. EBERs interact with several host proteins to form ribonucleoprotein (RNP) complexes. Although a precise function for EBERs remains elusive, roles in transformation and oncogenesis are proposed.
Epstein–Barr virus (EBV) latent membrane protein 2 (LMP2) are two viral proteins of the Epstein–Barr virus. LMP2A/LMP2B are transmembrane proteins that act to block tyrosine kinase signaling. LMP2A is a transmembrane protein that inhibits normal B-cell signal transduction by mimicking an activated B-cell receptor (BCR). The N-terminus domain of LMP2A is tyrosine phosphorylated and associates with Src family protein tyrosine kinases (PTKs) as well as spleen tyrosine kinase (Syk). PTKs and Syk are associated with BCR signal transduction.
The Epstein–Barr virus nuclear antigen 3 (EBNA-3) is a family of viral proteins associated with the Epstein–Barr virus. A typical EBV genome contains three such proteins:
The Epstein–Barr virus nuclear antigen 2 (EBNA-2) is one of the six EBV viral nuclear proteins expressed in latently infected B lymphocytes is a transactivator protein. EBNA2 is involved in the regulation of latent viral transcription and contributes to the immortalization of EBV infected cells. EBNA2 acts as an adapter molecule that binds to cellular sequence-specific DNA-binding proteins, JK recombination signal-binding protein (RBP-JK), and PU.1 as well as working with multiple members of the RNA polymerase II transcription complex.

The Herpesvirales is an order of dsDNA viruses with animal hosts, characterised by a common morphology consisting of an icosahedral capsid enclosed in a glycoprotein-containing lipid envelope. Common infections in humans caused by members of this order include cold sores, genital herpes, chickenpox, shingles, and glandular fever. Herpesvirales is the sole order in the class Herviviricetes, which is the sole class in the phylum Peploviricota.
Epstein–Barr virus stable intronic-sequence RNAs (ebv-sisRNAs) are a class of non-coding RNAs generated by repeat introns in the Epstein–Barr virus. After EBERs 1 and 2, ebv-sisRNA-1 is the third most abundant EBV RNA generated during a highly oncogenic form of virus latency. Conservation of ebv-sisRNA sequence and secondary structure between EBV and other herpesviruses suggest shared functions in latent infection.
Human herpes viruses, also known as HHVs, are part of a family of DNA viruses that cause several diseases in humans. One of the most notable functions of this virus family is their ability to enter a latent phase and lay dormant within animals for extended periods of time. The mechanism that controls this is very complex because expression of viral proteins during latency is decreased a great deal, meaning that the virus must have transcription of its genes repressed. There are many factors and mechanisms that control this process and epigenetics is one way this is accomplished. Epigenetics refers to persistent changes in expression patterns that are not caused by changes to the DNA sequence. This happens through mechanisms such as methylation and acetylation of histones, DNA methylation, and non-coding RNAs (ncRNA). Altering the acetylation of histones creates changes in expression by changing the binding affinity of histones to DNA, making it harder or easier for transcription machinery to access the DNA. Methyl and acetyl groups can also act as binding sites for transcription factors and enzymes that further modify histones or alter the DNA itself.
Gorilline gammaherpesvirus 1 (GoHV-1), commonly known as herpesvirus gorilla is a species of virus in the genus Lymphocryptovirus, subfamily Gammaherpesvirinae, family Herpesviridae, and order Herpesvirales.
Panine gammaherpesvirus 1 (PnHV-1), commonly known as chimpanzee lymphocryptovirus, is a species of virus in the genus Lymphocryptovirus, subfamily Gammaherpesvirinae, family Herpesviridae, and order Herpesvirales.
Papiine gammaherpesvirus 1 (PaHV-1), commonly known as baboon lymphocryptovirus, is a species of virus in the genus Lymphocryptovirus, subfamily Gammaherpesvirinae, family Herpesviridae, and order Herpesvirales.







