
Gedeon Richter Plc. is a European multinational pharmaceutical and biotechnology company headquartered in Budapest. It is the largest pharmaceutical company in Central and Eastern Europe, with an expanding direct presence in Western Europe, China, Northern America and Latin America. Richter has the largest R&D unit in Central and Eastern Europe and operations in over 100 countries.
INSIGHTEC Ltd., is a privately held medical device company that sells MR guided Focused Ultrasound equipment. The technology can destroy deep tissue in the body without the need for incisions.
Bial is a pharmaceutical company headquartered in São Mamede do Coronado, in Trofa, Porto district, Portugal. It was founded in 1924, being among the largest companies of its kind in Portugal. Its products are sold in pharmacies in more than 58 countries in 4 continents: Europe, America, Africa and Asia.

Gonadotropin-releasing hormone antagonists are a class of medications that antagonize the gonadotropin-releasing hormone receptor and thus the action of gonadotropin-releasing hormone (GnRH). They are used in the treatment of prostate cancer, endometriosis, uterine fibroids, female infertility in assisted reproduction, and for other indications.

Indiplon is a nonbenzodiazepine, hypnotic sedative that was developed in two formulations—an immediate-release formulation for sleep onset, and a modified-release version for sleep maintenance.

Cetrorelix, or cetrorelix acetate, sold under the brand name Cetrotide, is an injectable gonadotropin-releasing hormone (GnRH) antagonist. A synthetic decapeptide, it is used in assisted reproduction to inhibit premature luteinizing hormone surges The drug works by blocking the action of GnRH upon the pituitary, thus rapidly suppressing the production and action of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In addition, cetrorelix can be used to treat hormone-sensitive cancers of the prostate and breast and some benign gynaecological disorders. It is administered as either multiple 0.25 mg daily subcutaneous injections or as a single-dose 3 mg subcutaneous injection. The duration of the 3 mg single dose is four days; if human chorionic gonadotropin (hCG) is not administered within four days, a daily 0.25 mg dose is started and continued until hCG is administered.
MorphoSys AG is a German biopharmaceutical company founded in 1992. The company is headquartered near Munich, Germany, and has a wholly owned subsidiary, MorphoSys US Inc., in Boston, Massachusetts, in the US. The company has various antibody, protein and peptide technologies that it uses to discover and develop both proprietary and partnered drug candidates. The company has more than 100 drugs in its wider pipeline that are being investigated for a variety of diseases. While many of these are being developed in partnership with pharma and biotech companies, MorphoSys also has a proprietary pipeline with a focus on cancer and autoimmune diseases.

Ulipristal acetate, sold under the brand name Ella among others, is a medication used for emergency contraception and uterine fibroids. As emergency contraception it should be used within 120 hours of vaginally penetrating intercourse. For fibroids it may be taken for up to six months. It is taken by mouth.
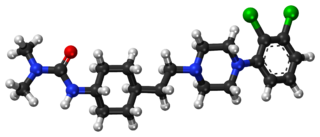
Cariprazine, sold under the brand names Vraylar, Reagila and Symvenu among others, is an atypical antipsychotic developed by Gedeon Richter, which is used in the treatment of schizophrenia, bipolar mania, bipolar depression, and major depressive disorder. It acts primarily as a D3 and D2 receptor partial agonist, with a preference for the D3 receptor. Cariprazine is also a partial agonist at the serotonin 5-HT1A receptor and acts as an antagonist at 5-HT2B and 5-HT2A receptors, with high selectivity for the D3 receptor. It is taken by mouth.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.

Filgotinib, sold under the brand name Jyseleca, is a medication used for the treatment of rheumatoid arthritis (RA). It was developed by the Belgian-Dutch biotech company Galapagos NV.

Elagolix, sold under the brand name Orilissa, is a gonadotropin-releasing hormone antagonist medication which is used in the treatment of pain associated with endometriosis in women. It is also under development for the treatment of uterine fibroids and heavy menstrual bleeding in women. The medication was under investigation for the treatment of prostate cancer and enlarged prostate in men as well, but development for these conditions was discontinued. Elagolix is taken by mouth once or twice per day. It can be taken for up to 6 to 24 months, depending on the dosage.

Relugolix, sold under the brand names Orgovyx and Relumina among others, is a gonadotropin-releasing hormone antagonist medication which is used in the treatment of prostate cancer in men and uterine fibroids in women. It is taken by mouth.
Allergan plc is an American, Irish-domiciled pharmaceutical company that acquires, develops, manufactures and markets brand name drugs and medical devices in the areas of medical aesthetics, eye care, central nervous system, and gastroenterology. The company is the maker of Botox.
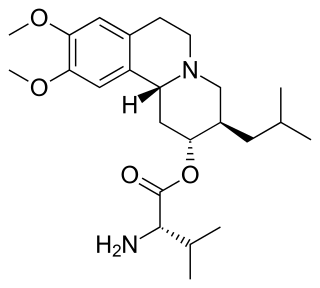
Valbenazine, sold under the brand name Ingrezza, is a medication used to treat tardive dyskinesia. It acts as a vesicular monoamine transporter 2 (VMAT2) inhibitor.
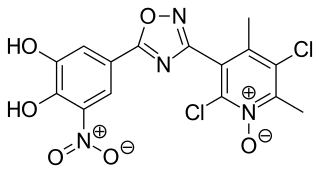
Opicapone, sold under the brand name Ongentys, is a medication which is administered together with levodopa in people with Parkinson's disease. Opicapone is a catechol-O-methyltransferase (COMT) inhibitor.
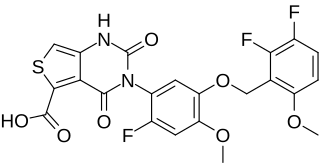
Linzagolix, sold under the brand name Yselty, is a medication used in the treatment of uterine fibroids. Linzagolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist developed by Kissei Pharmaceutical and ObsEva.
Elagolix/estradiol/norethisterone acetate, sold under the brand name Oriahnn, is a fixed-dose combination medication used to treat heavy menstrual bleeding associated with uterine leiomyomas (fibroids) in premenopausal women. It contains elagolix, a gonadotropin-releasing hormone (GnRH) receptor antagonist, estradiol, an estrogen, and norethisterone acetate, a progestin. It is taken by mouth. Oriahnn is co-packaged as a combination of elagolix/estradiol/norethisterone acetate capsules with elagolix capsules.

Relugolix/estradiol/norethisterone acetate, sold under the brand names Myfembree and Ryeqo, is a fixed-dose combination hormonal medication which is used for the treatment of heavy menstrual bleeding associated with uterine leiomyomas (fibroids) and for moderate to severe pain associated with endometriosis. It contains relugolix, an orally active gonadotropin-releasing hormone antagonist, estradiol, an estrogen, and norethisterone acetate, a progestin. The medication is taken by mouth.













