
Nicotiana is a genus of herbaceous plants and shrubs in the family Solanaceae, that is indigenous to the Americas, Australia, Southwestern Africa and the South Pacific. Various Nicotiana species, commonly referred to as tobacco plants, are cultivated as ornamental garden plants. N. tabacum is grown worldwide for the cultivation of tobacco leaves used for manufacturing and producing tobacco products, including cigars, cigarillos, cigarettes, chewing tobacco, dipping tobacco, snuff, and snus.

The Sphingidae are a family of moths commonly called sphinx moths, also colloquially known as hawk moths, with many of their caterpillars known as "hornworms"; it includes about 1,450 species. It is best represented in the tropics, but species are found in every region. They are moderate to large in size and are distinguished among moths for their agile and sustained flying ability, similar enough to that of hummingbirds as to be reliably mistaken for them. Their narrow wings and streamlined abdomens are adaptations for rapid flight. The family was named by French zoologist Pierre André Latreille in 1802.

A lima bean, also commonly known as the butter bean, sieva bean, double bean or Madagascar bean is a legume grown for its edible seeds or beans.

Manduca sexta is a moth of the family Sphingidae present through much of the Americas. The species was first described by Carl Linnaeus in his 1763 Centuria Insectorum.

Nicotiana rustica, commonly known as Aztec tobacco or strong tobacco, is a rainforest plant in the family Solanaceae. It is a very potent variety of tobacco, containing up to nine times more nicotine than common species of Nicotiana such as Nicotiana tabacum. More specifically, N. rustica leaves have a nicotine content as high as 9%, whereas N. tabacum leaves contain about 1 to 3%. The high concentration of nicotine in its leaves makes it useful for producing pesticides, and it has a wide variety of uses specific to cultures around the world. However, N. rustica is no longer cultivated in its native North America, as N. tabacum has replaced it.

Manduca quinquemaculata, the five-spotted hawkmoth, is a brown and gray hawk moth of the family Sphingidae. The caterpillar, often referred to as the tomato hornworm, can be a major pest in gardens; they get their name from a dark projection on their posterior end and their use of tomatoes as host plants. Tomato hornworms are closely related to the tobacco hornworm Manduca sexta. This confusion arises because caterpillars of both species have similar morphologies and feed on the foliage of various plants from the family Solanaceae, so either species can be found on tobacco or tomato leaves. Because of this, the plant on which the caterpillar is found does not indicate its species.

Nectar is a sugar-rich liquid produced by plants in glands called nectaries or nectarines, either within the flowers with which it attracts pollinating animals, or by extrafloral nectaries, which provide a nutrient source to animal mutualists, which in turn provide herbivore protection. Common nectar-consuming pollinators include mosquitoes, hoverflies, wasps, bees, butterflies and moths, hummingbirds, honeyeaters and bats. Nectar plays a crucial role in the foraging economics and evolution of nectar-eating species; for example, nectar foraging behavior is largely responsible for the divergent evolution of the African honey bee, A. m. scutellata and the western honey bee.

Plant defense against herbivory or host-plant resistance (HPR) is a range of adaptations evolved by plants which improve their survival and reproduction by reducing the impact of herbivores. Plants can sense being touched, and they can use several strategies to defend against damage caused by herbivores. Many plants produce secondary metabolites, known as allelochemicals, that influence the behavior, growth, or survival of herbivores. These chemical defenses can act as repellents or toxins to herbivores or reduce plant digestibility. Another defensive strategy of plants is changing their attractiveness. To prevent overconsumption by large herbivores, plants alter their appearance by changing their size or quality, reducing the rate at which they are consumed.

Nicotiana tabacum, or cultivated tobacco, is an annually grown herbaceous plant of the genus Nicotiana. N. tabacum is the most commonly grown species in the genus Nicotiana, as the plant's leaves are commercially harvested to be processed into tobacco for human use. The plant is tropical in origin, is commonly grown throughout the world, and is often found in cultivation. It grows to heights between 1 and 2 meters. Research is ongoing into its ancestry among wild Nicotiana species, but it is believed to be a hybrid of Nicotiana sylvestris, N. tomentosiformis, and possibly N. otophora.
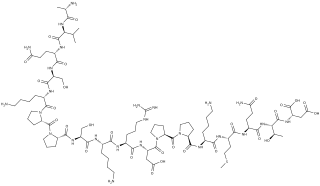
Systemin is a plant peptide hormone involved in the wound response in the family Solanaceae. It was the first plant hormone that was proven to be a peptide having been isolated from tomato leaves in 1991 by a group led by Clarence A. Ryan. Since then, other peptides with similar functions have been identified in tomato and outside of the Solanaceae. Hydroxyproline-rich glycopeptides were found in tobacco in 2001 and AtPeps were found in Arabidopsis thaliana in 2006. Their precursors are found both in the cytoplasm and cell walls of plant cells, upon insect damage, the precursors are processed to produce one or more mature peptides. The receptor for systemin was first thought to be the same as the brassinolide receptor but this is now uncertain. The signal transduction processes that occur after the peptides bind are similar to the cytokine-mediated inflammatory immune response in animals. Early experiments showed that systemin travelled around the plant after insects had damaged the plant, activating systemic acquired resistance, now it is thought that it increases the production of jasmonic acid causing the same result. The main function of systemins is to coordinate defensive responses against insect herbivores but they also affect plant development. Systemin induces the production of protease inhibitors which protect against insect herbivores, other peptides activate defensins and modify root growth. They have also been shown to affect plants' responses to salt stress and UV radiation. AtPEPs have been shown to affect resistance against oomycetes and may allow A. thaliana to distinguish between different pathogens. In Nicotiana attenuata, some of the peptides have stopped being involved in defensive roles and instead affect flower morphology.
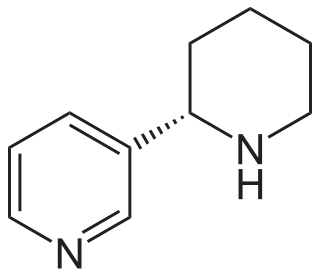
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco plant, as well as in the close relative of the common tobacco plant. It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide.

Leucyl aminopeptidases are enzymes that preferentially catalyze the hydrolysis of leucine residues at the N-terminus of peptides and proteins. Other N-terminal residues can also be cleaved, however. LAPs have been found across superkingdoms. Identified LAPs include human LAP, bovine lens LAP, porcine LAP, Escherichia coli LAP, and the solanaceous-specific acidic LAP (LAP-A) in tomato.

Manduca blackburni, the Hawaiian tomato hornworm, Hawaiian tobacco hornworm or Blackburn's sphinx moth, is a moth in the family Sphingidae.

Benzylacetone is a liquid with a sweet, flowery smell that is considered to be the most abundant attractant compound in flowers and one of volatile components of cocoa.
Plants and herbivores have co-evolved together for 350 million years. Plants have evolved many defense mechanisms against insect herbivory. Such defenses can be broadly classified into two categories: (1) permanent, constitutive defenses, and (2) temporary, inducible defenses. Both types are achieved through similar means but differ in that constitutive defenses are present before an herbivore attacks, while induced defenses are activated only when attacks occur. In addition to constitutive defenses, initiation of specific defense responses to herbivory is an important strategy for plant persistence and survival.
Green leaf volatiles (GLV) are organic compounds released by plants. Some of these chemicals function as signaling compounds between either plants of the same species, of other species, or even different lifeforms like insects.

Ian Thomas Baldwin is an American ecologist.

Tritrophic interactions in plant defense against herbivory describe the ecological impacts of three trophic levels on each other: the plant, the herbivore, and its natural enemies. They may also be called multitrophic interactions when further trophic levels, such as soil microbes, endophytes, or hyperparasitoids are considered. Tritrophic interactions join pollination and seed dispersal as vital biological functions which plants perform via cooperation with animals.
Bergamotenes are a group of isomeric chemical compounds with the molecular formula C15H24. The bergamotenes are found in a variety of plants, particularly in their essential oils.

Cannabis (/ˈkænəbɪs/) is commonly known as marijuana or hemp and has two known strains: Cannabis sativa and Cannabis indica, both of which produce chemicals to deter herbivory. The chemical composition includes specialized terpenes and cannabinoids, mainly tetrahydrocannabinol (THC), and cannabidiol (CBD). These substances play a role in defending the plant from pathogens including insects, fungi, viruses and bacteria. THC and CBD are stored mostly in the trichomes of the plant, and can cause psychological and physical impairment in the user, via the endocannabinoid system and unique receptors. THC increases dopamine levels in the brain, which attributes to the euphoric and relaxed feelings cannabis provides. As THC is a secondary metabolite, it poses no known effects towards plant development, growth, and reproduction. However, some studies show secondary metabolites such as cannabinoids, flavonoids, and terpenes are used as defense mechanisms against biotic and abiotic environmental stressors.

















