Anastrozole, sold under the brand name Arimidex among others, is a medication used in addition to other treatments for breast cancer. Specifically it is used for hormone receptor-positive breast cancer. It has also been used to prevent breast cancer in those at high risk. It is taken by mouth.

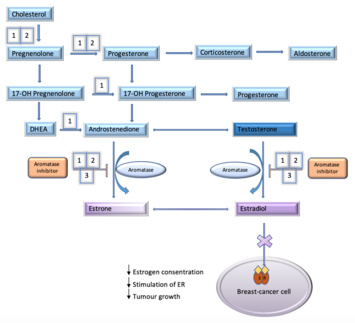
Aromatase, also called estrogen synthetase or estrogen synthase, is an enzyme responsible for a key step in the biosynthesis of estrogens. It is CYP19A1, a member of the cytochrome P450 superfamily, which are monooxygenases that catalyze many reactions involved in steroidogenesis. In particular, aromatase is responsible for the aromatization of androgens into estrogens. The enzyme aromatase can be found in many tissues including gonads, brain, adipose tissue, placenta, blood vessels, skin, and bone, as well as in tissue of endometriosis, uterine fibroids, breast cancer, and endometrial cancer. It is an important factor in sexual development.

Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men, and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.

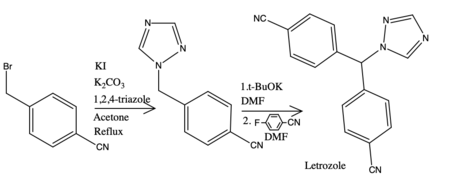
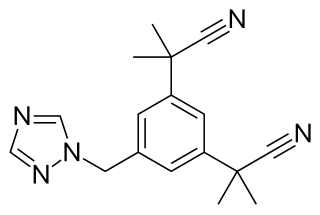
Letrozole, sold under the brand name Femara among others, is an aromatase inhibitor medication that is used in the treatment of breast cancer.

Aminoglutethimide (AG), sold under the brand names Elipten, Cytadren, and Orimeten among others, is a medication which has been used in the treatment of seizures, Cushing's syndrome, breast cancer, and prostate cancer, among other indications. It has also been used by bodybuilders, athletes, and other men for muscle-building and performance- and physique-enhancing purposes. AG is taken by mouth three or four times per day.
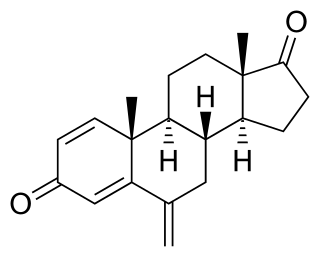
Exemestane, sold under the brand name Aromasin among others, is a medication used to treat breast cancer. It is a member of the class of antiestrogens known as aromatase inhibitors. Some breast cancers require estrogen to grow. Those cancers have estrogen receptors (ERs), and are called ER-positive. They may also be called estrogen-responsive, hormonally-responsive, or hormone-receptor-positive. Aromatase is an enzyme that synthesizes estrogen. Aromatase inhibitors block the synthesis of estrogen. This lowers the estrogen level, and slows the growth of cancers.
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents.

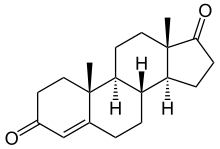
Formestane, formerly sold under the brand name Lentaron among others, is a steroidal, selective aromatase inhibitor which is used in the treatment of estrogen receptor-positive breast cancer in postmenopausal women. The drug is not active orally, and was available only as an intramuscular depot injection. Formestane was not approved by the United States FDA and the injectable form that was used in Europe in the past has been withdrawn from the market. Formestane is an analogue of androstenedione.
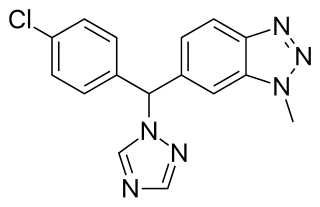
Vorozole is a triazole based competitive inhibitor of the aromatase enzyme. It underwent clinical testing for evaluation for use as an antineoplastic agent; however it was withdrawn from testing when no difference was detected in the duration of median survival as compared to the progestational agent megestrol acetate and research instead focused on the other third generation aromatase inhibitors anastrozole, letrozole and exemestane.
Hormonal therapy in oncology is hormone therapy for cancer and is one of the major modalities of medical oncology, others being cytotoxic chemotherapy and targeted therapy (biotherapeutics). It involves the manipulation of the endocrine system through exogenous or external administration of specific hormones, particularly steroid hormones, or drugs which inhibit the production or activity of such hormones. Because steroid hormones are powerful drivers of gene expression in certain cancer cells, changing the levels or activity of certain hormones can cause certain cancers to cease growing, or even undergo cell death. Surgical removal of endocrine organs, such as orchiectomy and oophorectomy can also be employed as a form of hormonal therapy.
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inhibiting or suppressing estrogen production. Antiestrogens are one of three types of sex hormone antagonists, the others being antiandrogens and antiprogestogens. Antiestrogens are commonly used to stop steroid hormones, estrogen, from binding to the estrogen receptors leading to the decrease of estrogen levels. Decreased levels of estrogen can lead to complications in sexual development. Antiandrogens are sex hormone antagonists which are able to lower the production and the effects that testosterone can have on female bodies.
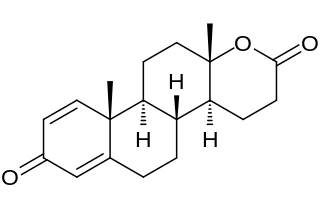
Testolactone is a non-selective, irreversible, steroidal aromatase inhibitor which is used as an antineoplastic drug to treat advanced-stage breast cancer. The drug was discontinued in 2008 and is no longer available for medical use.
A hormone-receptor-positive (HR+) tumor is a tumor which consists of cells that express receptors for certain hormones. The term most commonly refers to estrogen receptor positive tumors, but can also include progesterone receptor positive tumors. Estrogen-receptor-positive tumors depend on the presence of estrogen for ongoing proliferation.

Aromatase excess syndrome is a rare genetic and endocrine syndrome which is characterized by an overexpression of aromatase, the enzyme responsible for the biosynthesis of the estrogen sex hormones from the androgens, in turn resulting in excessive levels of circulating estrogens and, accordingly, symptoms of hyperestrogenism. It affects both sexes, manifesting itself in males as marked or complete phenotypical feminization and in females as hyperfeminization.

27-Hydroxycholesterol (27-HC) is an endogenous oxysterol with multiple biological functions, including activity as a selective estrogen receptor modulator (SERM) and as an agonist of the liver X receptor (LXR). It is a metabolite of cholesterol that is produced by the enzyme CYP27A1.

A nonsteroidal antiandrogen (NSAA) is an antiandrogen with a nonsteroidal chemical structure. They are typically selective and full or silent antagonists of the androgen receptor (AR) and act by directly blocking the effects of androgens like testosterone and dihydrotestosterone (DHT). NSAAs are used in the treatment of androgen-dependent conditions in men and women. They are the converse of steroidal antiandrogens (SAAs), which are antiandrogens that are steroids and are structurally related to testosterone.
Steroidal aromatase inhibitors are a class of drugs that are mostly used for treating breast cancer in postmenopausal women. High levels of estrogen in breast tissue increases the risk of developing breast cancer and the enzyme aromatase is considered to be a good therapeutic target when treating breast cancer due to it being involved in the final step of estrogen biosynthetic pathway and also its inhibition will not affect production of other steroids. Aromatase Inhibitors are classified into two categories based on their structure, nonsteroidal and steroidal; the latter resemble the structure of androstenedione. Steroidal aromatase inhibitors irreversibly inhibit the enzyme by binding covalently to the binding site of aromatase so the substrate cannot access it.
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.

The hydroxylation of estradiol is one of the major routes of metabolism of the estrogen steroid hormone estradiol. It is hydroxylated into the catechol estrogens 2-hydroxyestradiol and 4-hydroxyestradiol and into estriol (16α-hydroxyestradiol), reactions which are catalyzed by cytochrome P450 enzymes predominantly in the liver, but also in various other tissues.
Endocrine therapy is a common treatment for estrogen receptor positive breast cancer. However, resistance to this therapy can develop, leading to relapse and progression of disease. This highlights the need for new strategies to combat this resistance.