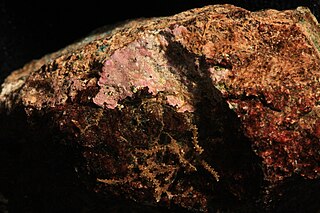An epiphyte is a plant or plant-like organism that grows on the surface of another plant and derives its moisture and nutrients from the air, rain, water or from debris accumulating around it. The plants on which epiphytes grow are called phorophytes. Epiphytes take part in nutrient cycles and add to both the diversity and biomass of the ecosystem in which they occur, like any other organism. They are an important source of food for many species. Typically, the older parts of a plant will have more epiphytes growing on them. Epiphytes differ from parasites in that they grow on other plants for physical support and do not necessarily affect the host negatively. An organism that grows on another organism that is not a plant may be called an epibiont. Epiphytes are usually found in the temperate zone or in the tropics. Epiphyte species make good houseplants due to their minimal water and soil requirements. Epiphytes provide a rich and diverse habitat for other organisms including animals, fungi, bacteria, and myxomycetes.

Brown algae are a large group of multicellular algae comprising the class Phaeophyceae. They include many seaweeds located in colder waters of the Northern Hemisphere. Brown algae are the major seaweeds of the temperate and polar regions. Many brown algae, such as members of the order Fucales, commonly grow along rocky seashores. Most brown algae live in marine environments, where they play an important role both as food and as a potential habitat. For instance, Macrocystis, a kelp of the order Laminariales, may reach 60 m (200 ft) in length and forms prominent underwater kelp forests that contain a high level of biodiversity. Another example is Sargassum, which creates unique floating mats of seaweed in the tropical waters of the Sargasso Sea that serve as the habitats for many species. Some members of the class, such as kelps, are used by humans as food.

In biology, a biological life cycle is a series of stages of the life of an organism, that begins as a zygote, often in an egg, and concludes as an adult that reproduces, producing an offspring in the form of a new zygote which then itself goes through the same series of stages, the process repeating in a cyclic fashion.

Hormosira is a genus of seaweed in the family Hormosiraceae. It is monotypic, with a single species, Hormosira banksii, also known as Neptune's necklace, Neptune's pearls, sea grapes, or bubbleweed it is native to Australia and New Zealand.

Codium is a genus of edible green macroalgae under the order Bryopsidales. The genus name is derived from a Greek word that pertains to the soft texture of its thallus. One of the foremost experts on Codium taxonomy was Paul Claude Silva at the University of California, Berkeley. P.C. Silva was able to describe 36 species for the genus and in honor of his work on Codium, the species C. silvae was named after the late professor.

Dictyotales is a large order in the brown algae containing the single family Dictyotaceae. Members of this order generally prefer warmer waters than other brown algae, and are prevalent in tropical and subtropical waters thanks to their many chemical defenses to ward off grazers. They display an isomorphic haplodiploid life cycle and are characterized by vegetative growth through a single apical cell. One genus in this order, Padina, is the only calcareous member of the brown algae.
In botany, a zoid or zoïd is a reproductive cell that possesses one or more flagella, and is capable of independent movement. Zoid can refer to either an asexually reproductive spore or a sexually reproductive gamete. In sexually reproductive gametes, zoids can be either male or female depending on the species. For example, some brown alga (Phaeophyceae) reproduce by producing multi-flagellated male and female gametes that recombine to form the diploid sporangia. Zoids are primarily found in some protists, diatoms, green alga, brown alga, non-vascular plants, and a few vascular plants. The most common classification group that produces zoids is the heterokonts or stramenopiles. These include green alga, brown alga, oomycetes, and some protists. The term is generally not used to describe motile, flagellated sperm found in animals. Zoid is also commonly confused for zooid which is a single organism that is part of a colonial animal.

Colpomenia is a genus of brown macroalgae in the family Scytosiphonaceae.

Blastocladiomycota is one of the currently recognized phyla within the kingdom Fungi. Blastocladiomycota was originally the order Blastocladiales within the phylum Chytridiomycota until molecular and zoospore ultrastructural characters were used to demonstrate it was not monophyletic with Chytridiomycota. The order was first erected by Petersen for a single genus, Blastocladia, which was originally considered a member of the oomycetes. Accordingly, members of Blastocladiomycota are often referred to colloquially as "chytrids." However, some feel "chytrid" should refer only to members of Chytridiomycota. Thus, members of Blastocladiomycota are commonly called "blastoclads" by mycologists. Alternatively, members of Blastocladiomycota, Chytridiomycota, and Neocallimastigomycota lumped together as the zoosporic true fungi. Blastocladiomycota contains 5 families and approximately 12 genera. This early diverging branch of kingdom Fungi is the first to exhibit alternation of generations. As well, two (once) popular model organisms—Allomyces macrogynus and Blastocladiella emersonii—belong to this phylum.

Dictyosphaeria is a genus of green algae in the family Siphonocladaceae.

Udotea is a genus of green algae in the family Udoteaceae.

Durvillaea is a genus of large brown algae in the monotypic family Durvillaeaceae. All members of the genus are found in the southern hemisphere, including Australia, New Zealand, South America, and various subantarctic islands. Durvillaea, commonly known as southern bull kelps, occur on rocky, wave-exposed shorelines and provide a habitat for numerous intertidal organisms. Many species exhibit a honeycomb-like structure in their fronds that provides buoyancy, which allows individuals detached from substrates to raft alive at sea, permitting dispersal for hundreds of days over thousands of kilometres. Durvillaea species have been used for clothing, tools and as a food source by many indigenous cultures throughout the South Pacific, and they continue to play a prominent role in Chilean cuisine.

Conceptacles are specialized cavities of marine and freshwater algae that contain the reproductive organs. They are situated in the receptacle and open by a small ostiole. Conceptacles are present in Corallinaceae, and Hildenbrandiales, as well as the brown Fucales. In the Fucales there is no haploid phase in the reproductive cycle and therefore no alternation of generations. The thallus is a sporophyte. The diploid plants produce male (antheridia) and female (oogonia) gametangia by meiosis. The gametes are released into the surrounding water; after fusion, the zygote settles and begins growth.
Korshikoviella is a genus of green algae in the family Characiaceae.

Turbinaria is a genus of brown algae (Phaeophyceae) found primarily in tropical marine waters. It generally grows on rocky substrates. In tropical Turbinaria species that are often preferentially consumed by herbivorous fishes and echinoids, there is a relatively low level of phenolics and tannins.
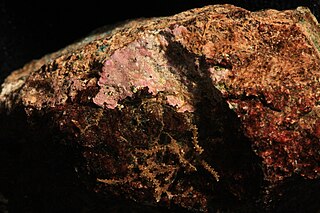
Hildenbrandia is a genus of thalloid red alga comprising about 26 species. The slow-growing, non-mineralized thalli take a crustose form. Hildenbrandia reproduces by means of conceptacles and produces tetraspores.
The epithallium or epithallus is the outer layer of a crustose coralline alga, which in some species is periodically shed to prevent organisms from attaching to and overgrowing the alga.

Gongolaria baccata is a species of brown seaweed in the family Fucaceae. It is found in the north east Atlantic, the Baltic Sea and the Mediterranean Sea. The species name baccata means "berry-like" and refers to the small air bladders.
Crustaphytum is a genus of red alga first discovered in Taoyuan algal reefs by Taiwanese scientists. The epithet “crusta” refers to crustose thallus and “phytum” refers to plant. Belonging to the family Hapalidiaceae in the order Hapalidiales, Crustaphytum is one kind of crustose coralline algae.

Amphiroa beauvoisii is a species of thalloid red algae in the Corallinaceae family. It is widely distributed across the world, and can be found attached to rocks in intertidal areas. Individual organisms consist of a base of calcified material, tissue in the shape of branching fan-like planes growing out of it. It exhibits a wide range of morphologies based on where it is found, as well as different reproductive behaviors based on season and location.