
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
Omega−3 fatty acids, also called Omega−3 oils, ω−3 fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond, three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.
Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health, but cannot synthesize them.
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.

Palmitic acid is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms. Its chemical formula is CH3(CH2)14COOH, and its C:D ratio is 16:0. It is a major component of palm oil from the fruit of Elaeis guineensis, making up to 44% of total fats. Meats, cheeses, butter, and other dairy products also contain palmitic acid, amounting to 50–60% of total fats.
In biochemistry and nutrition, a monounsaturated fat is a fat that contains a monounsaturated fatty acid (MUFA), a subclass of fatty acid characterized by having a double bond in the fatty acid chain with all of the remaining carbon atoms being single-bonded. By contrast, polyunsaturated fatty acids (PUFAs) have more than one double bond.

Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is a primary structural component of the human brain, cerebral cortex, skin, and retina. It is given the fatty acid notation 22:6(n-3). It can be synthesized from alpha-linolenic acid or obtained directly from maternal milk, fatty fish, fish oil, or algae oil. The consumption of DHA contributes to numerous physiological benefits, including cognition. As the primary structural component of nerve cells in the brain, the function of DHA is to support neuronal conduction and to allow optimal function of neuronal membrane proteins.

Palmitoleic acid, or (9Z)-hexadec-9-enoic acid, is an omega-7 monounsaturated fatty acid (16:1n-7) with the formula CH3(CH2)5CH=CH(CH2)7COOH. It is a rare component of fats. It is a common constituent of the glycerides of human adipose tissue. It is present in all tissues but, in general, found in higher concentrations in the liver.
Omega-9 fatty acids are a family of unsaturated fatty acids which have in common a final carbon–carbon double bond in the omega−9 position; that is, the ninth bond from the methyl end of the fatty acid.

In biochemistry and nutrition, a polyunsaturated fat is a fat that contains a polyunsaturated fatty acid, which is a subclass of fatty acid characterized by a backbone with two or more carbon–carbon double bonds. Some polyunsaturated fatty acids are essentials. Polyunsaturated fatty acids are precursors to and are derived from polyunsaturated fats, which include drying oils.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
A wax ester (WE) is an ester of a fatty acid and a fatty alcohol. Wax esters are the main components of three commercially important waxes: carnauba wax, candelilla wax, and beeswax.
Vaccenic acid is a naturally occurring trans fatty acid and an omega-7 fatty acid. It is the predominant kind of trans-fatty acid found in human milk, in the fat of ruminants, and in dairy products such as milk, butter, and yogurt. Trans fat in human milk may depend on trans fat content in food.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells. In addition to cytosolic fatty acid synthesis, there is also mitochondrial fatty acid synthesis (mtFASII), in which malonyl-CoA is formed from malonic acid with the help of malonyl-CoA synthetase (ACSF3), which then becomes the final product octanoyl-ACP (C8) via further intermediate steps.

Nervonic acid is a fatty acid. It is a monounsaturated analog of lignoceric acid (24:0). It is also known as selacholeic acid and cis-15-tetracosenoic acid. Its name derives from the Latin word nervus, meaning nerve or sinew.

Nannochloropsis is a genus of algae comprising six known species. The genus in the current taxonomic classification was first termed by Hibberd (1981). The species have mostly been known from the marine environment but also occur in fresh and brackish water. All of the species are small, nonmotile spheres which do not express any distinct morphological features that can be distinguished by either light or electron microscopy. The characterisation is mostly done by rbcL gene and 18S rRNA sequence analysis.
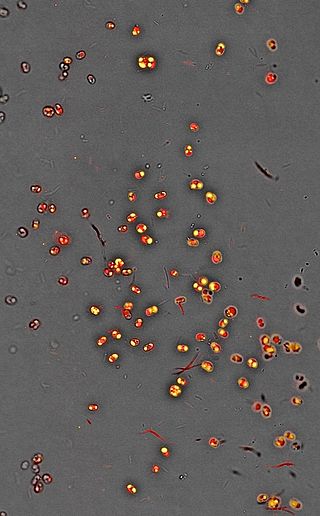
Nannochloropsis is a genus of alga within the heterokont line of eukaryotes, that is being investigated for biofuel production. One marine Nannochloropsis species has been shown to be suitable for algal biofuel production due to its ease of growth and high oil content, mainly unsaturated fatty acids and a significant percentage of palmitic acid. It also contains enough unsaturated fatty acid linolenic acid and polyunsaturated acid for a quality biodiesel.
Only two essential fatty acids are known to be essential for humans: alpha-linolenic acid and linoleic acid. The biological effects of the ω-3 and ω-6 fatty acids are mediated by their mutual interactions. Closely related, these fatty acids act as competing substrates for the same enzymes. The biological effects of the ω-3 and ω-6 fatty acids are largely mediated by essential fatty acid interactions. The proportion of omega-3 to omega-6 fatty acids in a diet may have metabolic consequences. Unlike omega-3 fatty acids and omega-6 fatty acids, omega-9 fatty acids are not classed as essential fatty acids because they can be created by the human body from monounsaturated and saturated fatty acids, and are therefore not essential in the diet.









