
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.
Omega−3 fatty acids, also called Omega−3 oils, ω−3 fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond, three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.

α-Linolenic acid, also known as alpha-Linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond.

Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the Neo-Latin word arachis (peanut), but peanut oil does not contain any arachidonic acid.

Omega-6 fatty acids are a family of polyunsaturated fatty acids that have in common a final carbon-carbon double bond in the n-6 position, that is, the sixth bond, counting from the methyl end.
gamma-Linolenic acid or GLA is an n−6, or omega-6, fatty acid found primarily in seed oils. When acting on GLA, arachidonate 5-lipoxygenase produces no leukotrienes and the conversion by the enzyme of arachidonic acid to leukotrienes is inhibited.
Linoleic acid (LA) is an organic compound with the formula HOOC(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.
Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5(n-3). It also has the trivial name timnodonic acid. In chemical structure, EPA is a carboxylic acid with a 20-carbon chain and five cis double bonds; the first double bond is located at the third carbon from the omega end.

Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is a primary structural component of the human brain, cerebral cortex, skin, and retina. In physiological literature, it is given the name 22:6(n-3). It can be synthesized from alpha-linolenic acid or obtained directly from maternal milk, fatty fish, fish oil, or algae oil.

In biochemistry and nutrition, a polyunsaturated fat is a fat that contains a polyunsaturated fatty acid, which is a subclass of fatty acid characterized by a backbone with two or more carbon–carbon double bonds. Some polyunsaturated fatty acids are essentials. Polyunsaturated fatty acids are precursors to and are derived from polyunsaturated fats, which include drying oils.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
Eicosatetraenoic acid (ETA) designates any straight chain 20:4 fatty acid. Eicosatetraenoic acid belongs to the family of eicosanoids, molecules synthesized from oxidized polyunsaturated fatty acids (PUFAs) to mediate cell-cell communication. The eicosanoids, working in tandem, contribute to a lipid signaling complex widely responsible for inducing an inflammatory immune response. Common signs of inflammation are both internal and external, with effects like visible redness, pain in the surrounding area, swelling, and the sensation of heat—many of these an effect of varying eicosanoid species. These effects are associated with and have been observed in patients with cancers and various neurological/metabolic disorders.
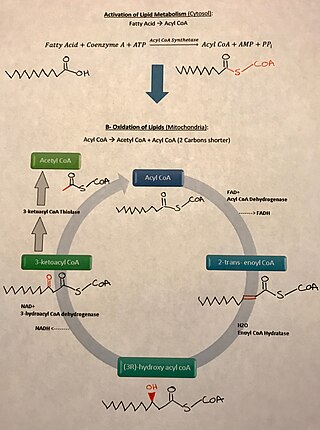
There are many fatty acids found in nature. Two types of fatty acids considered essential for human health are the omega-3 and omega-6 types. These two essential fatty acids are necessary for some cellular signalling pathways and are involved in mediating inflammation, protein synthesis, and metabolic pathways in the human body.
Docosapentaenoic acid (DPA) designates any straight open chain polyunsaturated fatty acid (PUFA) which contains 22 carbons and 5 double bonds. DPA is primarily used to designate two isomers, all-cis-4,7,10,13,16-docosapentaenoic acid and all-cis-7,10,13,16,19-docosapentaenoic acid. They are also commonly termed n-6 DPA and n-3 DPA, respectively; these designations describe the position of the double bond being 6 or 3 carbons closest to the (omega) carbon at the methyl end of the molecule and is based on the biologically important difference that n-6 and n-3 PUFA are separate PUFA classes, i.e. the omega-6 fatty acids and omega-3 fatty acids, respectively. Mammals, including humans, can not interconvert these two classes and therefore must obtain dietary essential PUFA fatty acids from both classes in order to maintain normal health.

Mead acid is an omega-9 fatty acid, first characterized by James F. Mead. As with some other omega-9 polyunsaturated fatty acids, animals can make Mead acid de novo. Its elevated presence in the blood is an indication of essential fatty acid deficiency. Mead acid is found in large quantities in cartilage.

Linoleoyl-CoA desaturase (also Delta 6 desaturase, EC 1.14.19.3) is an enzyme that converts between types of fatty acids, which are essential nutrients in the human body. The enzyme mainly catalyzes the chemical reaction
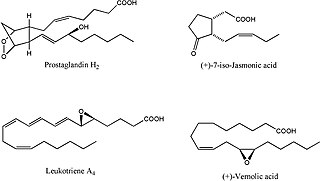
Oxylipins constitute a family of oxygenated natural products which are formed from fatty acids by pathways involving at least one step of dioxygen-dependent oxidation. Oxylipins are derived from polyunsaturated fatty acids (PUFAs) by COX enzymes (cyclooxygenases), by LOX enzymes (lipoxygenases), or by cytochrome P450 epoxygenase.
Only two essential fatty acids are known to be essential for humans: alpha-linolenic acid and linoleic acid. The biological effects of the ω-3 and ω-6 fatty acids are mediated by their mutual interactions. Closely related, these fatty acids act as competing substrates for the same enzymes. The biological effects of the ω-3 and ω-6 fatty acids are largely mediated by essential fatty acid interactions. The proportion of omega-3 to omega-6 fatty acids in a diet may have metabolic consequences. Unlike omega-3 fatty acids and omega-6 fatty acids, omega-9 fatty acids are not classed as essential fatty acids because they can be created by the human body from monounsaturated and saturated fatty acids, and are therefore not essential in the diet.











