The Grignard reaction is an organometallic chemical reaction in which carbon alkyl, allyl, vinyl, or aryl magnesium halides are added to the carbonyl groups of either an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds.

In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word synthon has now come to be used to mean synthetic building block rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book The Logic of Chemical Synthesis, as it was not included in the index. Because synthons are charged, when placed into a synthesis an uncharged form is found commercially instead of forming and using the potentially very unstable charged synthons.
Metalation is a chemical reaction that forms a bond to a metal. This reaction usually refers to the replacement of a halogen atom in an organic molecule with a metal atom, resulting in an organometallic compound. In the laboratory, metalation is commonly used to activate organic molecules during the formation of C—X bonds, which are necessary for the synthesis of many organic molecules.
The Hiyama coupling is a palladium-catalyzed cross-coupling reaction of organosilanes with organic halides used in organic chemistry to form carbon–carbon bonds. This reaction was discovered in 1988 by Tamejiro Hiyama and Yasuo Hatanaka as a method to form carbon-carbon bonds synthetically with chemo- and regioselectivity. The Hiyama coupling has been applied to the synthesis of various natural products.
The Corey–House synthesis (also called the Corey–Posner–Whitesides–House reaction and other permutations) is an organic reaction that involves the reaction of a lithium diorganylcuprate () with an organic halide or pseudohalide () to form a new alkane, as well as an ill-defined organocopper species and lithium (pseudo)halide as byproducts.

The Barbier reaction is an organometallic reaction between an alkyl halide, a carbonyl group and a metal. The reaction can be performed using magnesium, aluminium, zinc, indium, tin, samarium, barium or their salts. The reaction product is a primary, secondary or tertiary alcohol. The reaction is similar to the Grignard reaction but the crucial difference is that the organometallic species in the Barbier reaction is generated in situ, whereas a Grignard reagent is prepared separately before addition of the carbonyl compound. Unlike many Grignard reagents, the organometallic species generated in a Barbier reaction are unstable and thus cannot be stored or sold commercially. Barbier reactions are nucleophilic addition reactions that involve relatively inexpensive, water insensitive metals or metal compounds. For this reason, it is possible in many cases to run the reaction in water, making the procedure part of green chemistry. In contrast, Grignard reagents and organolithium reagents are highly moisture sensitive and must be used under an inert atmosphere without the presence of water. The Barbier reaction is named after Philippe Barbier, who was Victor Grignard's teacher.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
The Blaise ketone synthesis is the chemical reaction of acid chlorides with organozinc compounds to give ketones.

A Grignard reagent or Grignard compound is a chemical compound with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Methyllithium is the simplest organolithium reagent, with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.

A Rieke metal is a highly reactive metal powder generated by reduction of a metal salt with an alkali metal. These materials are named after Reuben D. Rieke, who first described the recipes for their preparation. Among the many metals that have been generated by this method are Mg, Ca, Ti, Fe, Co, Ni, Cu, Zn, and In, which in turn are called Rieke-magnesium, Rieke-calcium, etc.
The Fukuyama coupling is a coupling reaction taking place between a thioester and an organozinc halide in the presence of a palladium catalyst. The reaction product is a ketone. This reaction was discovered by Tohru Fukuyama et al. in 1998.

The Nozaki–Hiyama–Kishi reaction is a nickel/chromium coupling reaction forming an alcohol from the reaction of an aldehyde with an allyl or vinyl halide. In their original 1977 publication, Tamejiro Hiyama and Hitoshi Nozaki reported on a chromium(II) salt solution prepared by reduction of chromic chloride by lithium aluminium hydride to which was added benzaldehyde and allyl chloride:

Tetramethyltin is an organometallic compound with the formula (CH3)4Sn. This liquid, one of the simplest organotin compounds, is useful for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. It is volatile and toxic, so care should be taken when using it in the laboratory.
Organomanganese chemistry is the chemistry of organometallic compounds containing a carbon to manganese chemical bond. In a 2009 review, Cahiez et al. argued that as manganese is cheap and benign, organomanganese compounds have potential as chemical reagents, although currently they are not widely used as such despite extensive research.
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen.
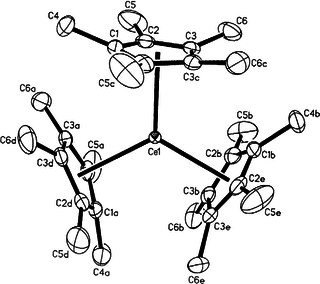
Organocerium chemistry is the science exploring the properties, structure, and reactivity of organocerium compounds, chemical compounds that contain one or more chemical bond between carbon and cerium. These compounds comprise a subset of the organolanthanides. In general, organocerium compounds are not isolable, and are rather studied in solution via their reactions with other species. There are notable exceptions, such as the Cp*3Ce(III) complex shown at right, but they are relatively rare. Complexes involving cerium of various oxidation states are known: though lanthanides are most stable in the +3 state, complexes of cerium(IV) have been reported. These latter compounds have found less widespread use due to their oxidizing nature, and the majority of literature regarding organometallic cerium complexes involves the +3 oxidation state. In particular, organocerium compounds have been developed extensively as non-basic carbon nucleophiles in organic synthesis. Because cerium is relatively non-toxic, they serve as an "environmentally friendly" alternative to other organometallic reagents. Several reviews detailing these applications have been published.