Podoplanin is a protein that in humans is encoded by the PDPN gene. [5] [6] [7]
Podoplanin is a protein that in humans is encoded by the PDPN gene. [5] [6] [7]
Podoplanin is a mucin-type protein with a mass of 36- to 43-kDa. It is relatively well conserved between species, with homologues in humans, mice, rats, dogs and hamsters. [8]
This gene encodes a type-I, integral membrane, heavily O-glycosylated glycoprotein with diverse distribution in human tissues. The physiological function of this protein may be related to its mucin-type character. The homologous protein in other species has been described as a differentiation antigen and influenza-virus receptor. The specific function of this protein has not been determined but it has been proposed as a marker of lung injury. Alternatively spliced transcript variants encoding different isoforms have been identified. [7]
This protein has been found to have functions in lung alveolar cells, kidney podocytes, and lymphatic endothelial cells. More recently, this protein has been found in neural tissue in both mouse and human samples. [9]
In lymphatic endothelial cells, experimentation has indicated that podoplanin plays a role in proper formation of linkages between the cardiovascular system and the lymphatic systems, typically causing fatty liver disease in these mice. [9]
Although the exact function is unknown in many tissues, podoplanin is generally receptive to detection via immunofluorescent staining and has been shown to co-localize with the protein nestin, a type VI intermediate filament protein expressed almost primarily in neural tissues. [10] Currently, the only protein known to interact with podoplanin physiologically is CLEC-2, a C-type lectin 2 expressed on platelets and on hematopoietic cells. [11] Both serve a role in the proper formation of blood/lymphatic connections in embryonic development.
PDPN has been studied extensively in the cancer field. It is a specific lymphatic vessel marker, and since lymphangiogenesis levels are correlated with poor prognosis in cancer patients, it can be used as a diagnostic marker. [8] It is often upregulated in certain types of cancer, including several types of squamous cell carcinomas, malignant mesothelioma and brain tumors. [8] Moreover, it can be upregulated by cancer-associated fibroblasts (CAFs) in the tumor stroma, [8] [12] where it has been associated with poor prognosis. [13]
In squamous cell carcinomas, PDPN is believed to play a key role in the cancer cell invasiveness by controlling invadopodia, and thus mediating efficient ECM degradation. [14]

The endothelium is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the rest of the vessel wall. Endothelial cells form the barrier between vessels and tissue and control the flow of substances and fluid into and out of a tissue.
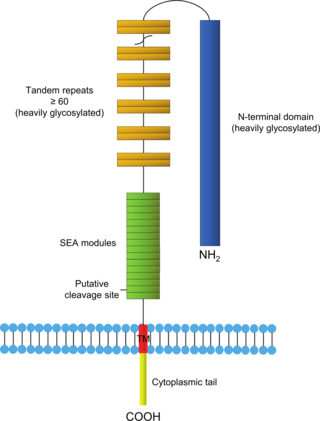
Mucin-16(MUC-16) also known as Ovarian cancer-related tumor marker CA125 is a protein that in humans is encoded by the MUC16 gene. MUC-16 is a member of the mucin family glycoproteins. MUC-16 has found application as a tumor marker or biomarker that may be elevated in the blood of some patients with specific types of cancers, most notably ovarian cancer, or other conditions that are benign.

The selectins are a family of cell adhesion molecules. All selectins are single-chain transmembrane glycoproteins that share similar properties to C-type lectins due to a related amino terminus and calcium-dependent binding. Selectins bind to sugar moieties and so are considered to be a type of lectin, cell adhesion proteins that bind sugar polymers.
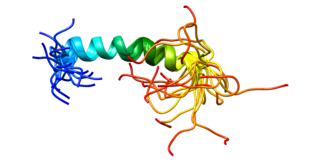
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is a protein that in humans is encoded by the PECAM1 gene found on chromosome17q23.3. PECAM-1 plays a key role in removing aged neutrophils from the body.

P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene.

Microvesicles are a type of extracellular vesicle (EV) that are released from the cell membrane. In multicellular organisms, microvesicles and other EVs are found both in tissues and in many types of body fluids. Delimited by a phospholipid bilayer, microvesicles can be as small as the smallest EVs or as large as 1000 nm. They are considered to be larger, on average, than intracellularly-generated EVs known as exosomes. Microvesicles play a role in intercellular communication and can transport molecules such as mRNA, miRNA, and proteins between cells.

Mucin short variant S1, also called polymorphic epithelial mucin (PEM) or epithelial membrane antigen (EMA), is a mucin encoded by the MUC1 gene in humans. Mucin short variant S1 is a glycoprotein with extensive O-linked glycosylation of its extracellular domain. Mucins line the apical surface of epithelial cells in the lungs, stomach, intestines, eyes and several other organs. Mucins protect the body from infection by pathogen binding to oligosaccharides in the extracellular domain, preventing the pathogen from reaching the cell surface. Overexpression of MUC1 is often associated with colon, breast, ovarian, lung and pancreatic cancers. Joyce Taylor-Papadimitriou identified and characterised the antigen during her work with breast and ovarian tumors.
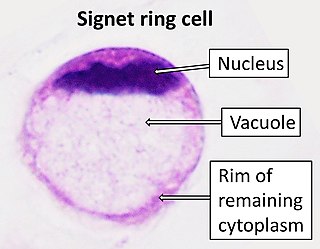
Signet ring cell carcinoma (SRCC) is a rare form of highly malignant adenocarcinoma that produces mucin. It is an epithelial malignancy characterized by the histologic appearance of signet ring cells.

CD47 also known as integrin associated protein (IAP) is a transmembrane protein that in humans is encoded by the CD47 gene. CD47 belongs to the immunoglobulin superfamily and partners with membrane integrins and also binds the ligands thrombospondin-1 (TSP-1) and signal-regulatory protein alpha (SIRPα). CD-47 acts as a don't eat me signal to macrophages of the immune system which has made it a potential therapeutic target in some cancers, and more recently, for the treatment of pulmonary fibrosis.

CD93 is a protein that in humans is encoded by the CD93 gene. CD93 is a C-type lectin transmembrane receptor which plays a role not only in cell–cell adhesion processes but also in host defense.
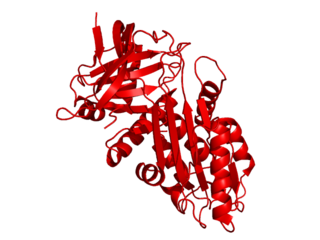
Serpin B3 is a protein that in humans is encoded by the SERPINB3 gene.

C-fos-induced growth factor (FIGF) is a vascular endothelial growth factor that in humans is encoded by the FIGF gene.

Prospero homeobox protein 1 is a protein that in humans is encoded by the PROX1 gene. The Prox1 gene is critical for the development of multiple tissues. Prox1 activity is necessary and sufficient to specify a lymphatic endothelial cell fate in endothelial progenitors located in the embryonic veins.

CD226, PTA1 or DNAM-1 is a ~65 kDa immunoglobulin-like transmembrane glycoprotein expressed on the surface of natural killer cells, NK T cell, B cells, dendritic cells, hematopoietic precursor cells, platelets, monocytes and T cells.

Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1), also known as extracellular link domain containing 1 (XLKD1) is a Link domain-containing hyaladherin, a protein capable of binding to hyaluronic acid (HA), homologous to CD44, the main HA receptor. In humans it is encoded by the LYVE1 gene.

DCN1-like protein 1 is a protein that in humans is encoded by the DCUN1D1 gene.
The Lymphatic Endothelium refers to a specialized subset of endothelial cells located in the sinus systems of draining lymph nodes. Specifically, these endothelial cells line the branched sinus systems formed by afferent lymphatic vessels, forming a single-cell layer which functions in a variety of critical physiological processes. These lymphatic endothelial cells contribute directly to immune function and response modulation, provide transport selectivity, and demonstrate orchestration of bidirectional signaling cascades. Additionally, lymphatic endothelial cells may be implicated in downstream immune cell development as well as lymphatic organogenesis. Until recently, lymphatic endothelial cells have not been characterized to their optimal potential. This system is very important in the function of continuous removal of interstitial fluid and proteins, while also having a significant function of entry for leukocytes and tumor cells. This leads to further research that is being developed on the relationship between lymphatic endothelium and metastasis of tumor cells . The lymphatic capillaries are described to be blind ended vessels, and they are made up of a single non-fenestrated layer of endothelial cells; The lymph capillaries function to aid in the uptake of fluids, macromolecules, and cells. Although they are generally similar to blood capillaries, the lymph capillaries have distinct structural differences. Lymph capillaries consist of a more wide and irregular lumen, and the endothelium in lymph capillaries is much thinner as well. Their origin has been speculated to vary based on them being dependent on specific tissue environments, and powered by organ-specific signals.(L. Gutierrez-Miranda, K. Yaniv, 2020). A lymph capillary endothelial cell is distinct from other endothelial cells in that collagen fibers are directly attached to its plasma membrane.
Large cell lung carcinoma with rhabdoid phenotype (LCLC-RP) is a rare histological form of lung cancer, currently classified as a variant of large cell lung carcinoma (LCLC). In order for a LCLC to be subclassified as the rhabdoid phenotype variant, at least 10% of the malignant tumor cells must contain distinctive structures composed of tangled intermediate filaments that displace the cell nucleus outward toward the cell membrane. The whorled eosinophilic inclusions in LCLC-RP cells give it a microscopic resemblance to malignant cells found in rhabdomyosarcoma (RMS), a rare neoplasm arising from transformed skeletal muscle. Despite their microscopic similarities, LCLC-RP is not associated with rhabdomyosarcoma.

In molecular biology miR-205 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. They are involved in numerous cellular processes, including development, proliferation, and apoptosis. Currently, it is believed that miRNAs elicit their effect by silencing the expression of target genes.

Melanoma inhibitory activity protein 3 (MIA3), also known as transport and Golgi organization protein 1 (TANGO1), is a protein that in humans is encoded by the MIA3 gene on chromosome 1. It is ubiquitously expressed in many tissues and cell types. MIA3 localizes to the endoplasmic reticulum (ER) exit site, where it binds bulky cargo molecules such as collagens and creates mega transport carriers for the export of cargoes from the ER. This function suggests that it plays a role in assembly of extracellular matrix (ECM) and bone formation. MIA3 has been demonstrated to contribute to both tumor suppression and progression. The MIA3 gene also contains one of 27 loci associated with increased risk of coronary artery disease.. A TANGO1 like protein called TALI is expressed in liver and intestine and shown to be required for the export of bulky very Low density lipoproteins (VLDL) and chylomicrons. TANGO1 and TALI assemble into rings around COPII coats and this function is necessary for export of bulky cargoes. The discovery of TANGO1 and understanding its function has revealed that cargo export from the ER is not be vesicles but involves transient tunnels between the ER exit site and the next compartment of the secretory pathway. Biallelic Mutations in TANGO1 cause syndrome disease and complete loss of TANGO1 leads of defects in bone mineralization. These findings highlight the significance of TANGO1 in building and ER exit site, controlling the quantities and quality of cargo exported, which is necessary for life.