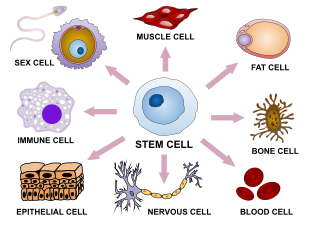
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. However, metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.
Internexin, alpha-internexin, is a Class IV intermediate filament approximately 66 KDa. The protein was originally purified from rat optic nerve and spinal cord. The protein copurifies with other neurofilament subunits, as it was originally discovered, however in some mature neurons it can be the only neurofilament expressed. The protein is present in developing neuroblasts and in the central nervous system of adults. The protein is a major component of the intermediate filament network in small interneurons and cerebellar granule cells, where it is present in the parallel fibers.

Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks, but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the PRPH gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despite extensive research into how neurofilaments and peripherin contribute to ALS, their role in this disease is still unidentified.
Glial fibrillary acidic protein (GFAP) is a protein that is encoded by the GFAP gene in humans. It is a type III intermediate filament (IF) protein that is expressed by numerous cell types of the central nervous system (CNS), including astrocytes and ependymal cells during development. GFAP has also been found to be expressed in glomeruli and peritubular fibroblasts taken from rat kidneys, Leydig cells of the testis in both hamsters and humans, human keratinocytes, human osteocytes and chondrocytes and stellate cells of the pancreas and liver in rats.
Neurofilaments (NF) are classed as type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with microtubules (~25 nm) and microfilaments (7 nm), they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into six types based on their gene organization and protein structure. Types I and II are the keratins which are expressed in epithelia. Type III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Type IV consists of the neurofilament proteins NF-L, NF-M, NF-H and α-internexin. Type V consists of the nuclear lamins, and type VI consists of the protein nestin. The type IV intermediate filament genes all share two unique introns not found in other intermediate filament gene sequences, suggesting a common evolutionary origin from one primitive type IV gene.
Neuroepithelial cells, or neuroectodermal cells, form the wall of the closed neural tube in early embryonic development. The neuroepithelial cells span the thickness of the tube's wall, connecting with the pial surface and with the ventricular or lumenal surface. They are joined at the lumen of the tube by junctional complexes, where they form a pseudostratified layer of epithelium called neuroepithelium.
Neural stem cells (NSCs) are self-renewing, multipotent cells that firstly generate the radial glial progenitor cells that generate the neurons and glia of the nervous system of all animals during embryonic development. Some neural progenitor stem cells persist in highly restricted regions in the adult vertebrate brain and continue to produce neurons throughout life. Differences in the size of the central nervous system are among the most important distinctions between the species and thus mutations in the genes that regulate the size of the neural stem cell compartment are among the most important drivers of vertebrate evolution.

Bone morphogenetic protein 4 is a protein that in humans is encoded by BMP4 gene. BMP4 is found on chromosome 14q22-q23.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.
A neurosphere is a culture system composed of free-floating clusters of neural stem cells. Neurospheres provide a method to investigate neural precursor cells in vitro. Putative neural stem cells are suspended in a medium lacking adherent substrates but containing necessary growth factors, such as epidermal growth factor and fibroblast growth factor. This allows the neural stem cells to form into characteristic 3-D clusters. However, neurospheres are not identical to stem cells; rather, they only contain a small percentage of neural stem cells.

A glial scar formation (gliosis) is a reactive cellular process involving astrogliosis that occurs after injury to the central nervous system. As with scarring in other organs and tissues, the glial scar is the body's mechanism to protect and begin the healing process in the nervous system.

Transcription factor HES1 is a protein that is encoded by the Hes1 gene, and is the mammalian homolog of the hairy gene in Drosophila. HES1 is one of the seven members of the Hes gene family (HES1-7). Hes genes code nuclear proteins that suppress transcription.
Neurogenins, often abbreviated as Ngn, are a family of bHLH transcription factors involved in specifying neuronal differentiation. The family consisting of Neurogenin-1, Neurogenin-2, and Neurogenin-3, plays a fundamental role in specifying neural precursor cells and regulating the differentiation of neurons during embryonic development. It is one of many gene families related to the atonal gene in Drosophila. Other positive regulators of neuronal differentiation also expressed during early neural development include NeuroD and ASCL1.

Neurofilament light polypeptide, also known as neurofilament light chain, abbreviated to NF-L or Nfl and with the HGNC name NEFL is a member of the intermediate filament protein family. This protein family consists of over 50 human proteins divided into 5 major classes, the Class I and II keratins, Class III vimentin, GFAP, desmin and the others, the Class IV neurofilaments and the Class V nuclear lamins. There are four major neurofilament subunits, NF-L, NF-M, NF-H and α-internexin. These form heteropolymers which assemble to produce 10nm neurofilaments which are only expressed in neurons where they are major structural proteins, particularly concentrated in large projection axons. Axons are particularly sensitive to mechanical and metabolic compromise and as a result axonal degeneration is a significant problem in many neurological disorders. The detection of neurofilament subunits in CSF and blood has therefore become widely used as a biomarker of ongoing axonal compromise. The NF-L protein is encoded by the NEFL gene. Neurofilament light chain is a biomarker that can be measured with immunoassays in cerebrospinal fluid and plasma and reflects axonal damage in a wide variety of neurological disorders. It is a useful marker for disease monitoring in amyotrophic lateral sclerosis, multiple sclerosis, Alzheimer's disease, and more recently Huntington's disease. It is also promising marker for follow-up of patients with brain tumors. Higher levels of blood or CSF NF-L have been associated with increased mortality, as would be expected as release of this protein reflects ongoing axonal loss. Recent work performed as a collaboration between EnCor Biotechnology Inc. and the University of Florida showed that the NF-L antibodies employed in the most widely used NF-L assays are specific for cleaved forms of NF-L generated by proteolysis induced by cell death. Methods used in different studies for NfL measurement are sandwich enzyme-linked immunosorbent assay (ELISA), electrochemiluminescence, and high-sensitive single molecule array (SIMOA).
Stem cell markers are genes and their protein products used by scientists to isolate and identify stem cells. Stem cells can also be identified by functional assays. Below is a list of genes/protein products that can be used to identify various types of stem cells, or functional assays that do the same. The initial version of the list below was obtained by mining the PubMed database as described in

Neurogenin-2 is a protein that in humans is encoded by the NEUROG2 gene.

A neuronal lineage marker is an endogenous tag that is expressed in different cells along neurogenesis and differentiated cells such as neurons. It allows detection and identification of cells by using different techniques. A neuronal lineage marker can be either DNA, mRNA or RNA expressed in a cell of interest. It can also be a protein tag, as a partial protein, a protein or an epitope that discriminates between different cell types or different states of a common cell. An ideal marker is specific to a given cell type in normal conditions and/or during injury. Cell markers are very valuable tools for examining the function of cells in normal conditions as well as during disease. The discovery of various proteins specific to certain cells led to the production of cell-type-specific antibodies that have been used to identify cells.
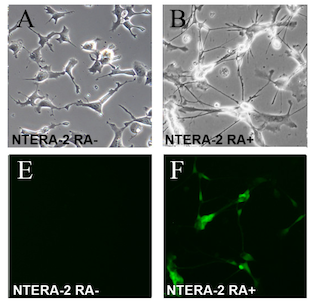
The NTERA-2 cell line is a clonally derived, pluripotent human embryonal carcinoma cell line.

Musashi-2, also known as Musashi RNA binding protein 2, is a protein that in humans is encoded by the MSI2 gene. Like its homologue musashi-1 (MSI1), it is an RNA-binding protein involved in stemness.















