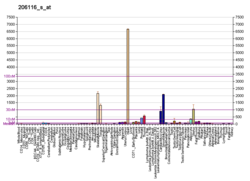
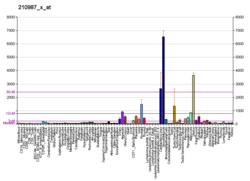
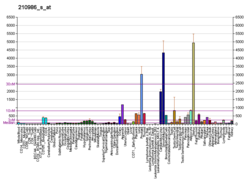
Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. [5] This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.