Related Research Articles

Microtubules are polymers of tubulin that form part of the cytoskeleton and provide structure and shape to eukaryotic cells. Microtubules can be as long as 50 micrometres, as wide as 23 to 27 nm and have an inner diameter between 11 and 15 nm. They are formed by the polymerization of a dimer of two globular proteins, alpha and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule. The most common form of a microtubule consists of 13 protofilaments in the tubular arrangement.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components:microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.

Tubulin in molecular biology can refer either to the tubulin protein superfamily of globular proteins, or one of the member proteins of that superfamily. α- and β-tubulins polymerize into microtubules, a major component of the eukaryotic cytoskeleton. Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for DNA segregation and therefore cell division.

The tau proteins are a group of six highly soluble protein isoforms produced by alternative splicing from the gene MAPT. They have roles primarily in maintaining the stability of microtubules in axons and are abundant in the neurons of the central nervous system (CNS), where the cerebral cortex has the highest abundance. They are less common elsewhere but are also expressed at very low levels in CNS astrocytes and oligodendrocytes.

Stathmin, also known as metablastin and oncoprotein 18 is a protein that in humans is encoded by the STMN1 gene.
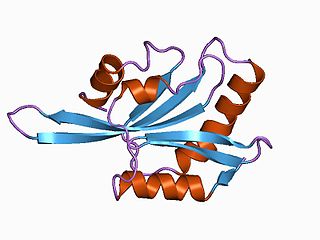
ADF/cofilin is a family of actin-binding proteins associated with the rapid depolymerization of actin microfilaments that give actin its characteristic dynamic instability. This dynamic instability is central to actin's role in muscle contraction, cell motility and transcription regulation.
A neurite or neuronal process refers to any projection from the cell body of a neuron. This projection can be either an axon or a dendrite. The term is frequently used when speaking of immature or developing neurons, especially of cells in culture, because it can be difficult to tell axons from dendrites before differentiation is complete.
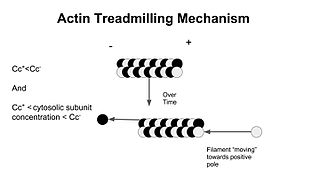
In molecular biology, treadmilling is a phenomenon observed within protein filaments of the cytoskeletons of many cells, especially in actin filaments and microtubules. It occurs when one end of a filament grows in length while the other end shrinks, resulting in a section of filament seemingly "moving" across a stratum or the cytosol. This is due to the constant removal of the protein subunits from these filaments at one end of the filament, while protein subunits are constantly added at the other end. Treadmilling was discovered by Wegner, who defined the thermodynamic and kinetic constraints. Wegner recognized that: “The equilibrium constant (K) for association of a monomer with a polymer is the same at both ends, since the addition of a monomer to each end leads to the same polymer.”; a simple reversible polymer can’t treadmill; ATP hydrolysis is required. GTP is hydrolyzed for microtubule treadmilling.

In biology, a protein filament is a long chain of protein monomers, such as those found in hair, muscle, or in flagella. Protein filaments form together to make the cytoskeleton of the cell. They are often bundled together to provide support, strength, and rigidity to the cell. When the filaments are packed up together, they are able to form three different cellular parts. The three major classes of protein filaments that make up the cytoskeleton include: actin filaments, microtubules and intermediate filaments.

Microtubule-associated protein 2 is a protein in humans that is encoded by the MAP2 gene.

Microtubule-associated protein 4 is a protein that in humans is encoded by the MAP4 gene.

Serine/threonine-protein kinase MARK1 is an enzyme that in humans is encoded by the MARK1 gene.

Microtubule-actin cross-linking factor 1, isoforms 1/2/3/5 is a protein that in humans is encoded by the MACF1 gene.

Cyclin-dependent kinase 5 is a protein, and more specifically an enzyme, that is encoded by the Cdk5 gene. It was discovered 15 years ago, and it is saliently expressed in post-mitotic central nervous system neurons (CNS).
Collapsin response mediator protein family or CRMP family consists of five intracellular phosphoproteins of similar molecular size and high (50–70%) amino acid sequence identity. CRMPs are predominantly expressed in the nervous system during development and play important roles in axon formation from neurites and in growth cone guidance and collapse through their interactions with microtubules. Cleaved forms of CRMPs have also been linked to neuron degeneration after trauma induced injury.
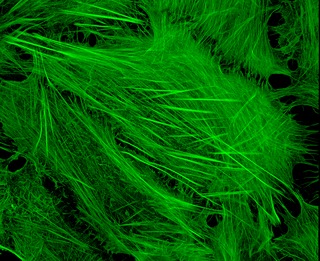
Cytoskeletal drugs are small molecules that interact with actin or tubulin. These drugs can act on the cytoskeletal components within a cell in three main ways. Some cytoskeletal drugs stabilize a component of the cytoskeleton, such as taxol, which stabilizes microtubules, or Phalloidin, which stabilizes actin filaments. Others, such as Cytochalasin D, bind to actin monomers and prevent them from polymerizing into filaments. Drugs such as demecolcine act by enhancing the depolymerisation of already formed microtubules. Some of these drugs have multiple effects on the cytoskeleton: for example, Latrunculin both prevents actin polymerization as well as enhancing its rate of depolymerization. Typically the microtubule targeting drugs can be found in the clinic where they are used therapeutically in the treatment of some forms of cancer. As a result of the lack of specificity for specific type of actin, the use of these drugs in animals results in unacceptable off-target effects. Despite this, the actin targeting compounds are still useful tools that can be used on a cellular level to help further our understanding of how this complex part of the cells' internal machinery operates. For example, Phalloidin that has been conjugated with a fluorescent probe can be used for visualizing the filamentous actin in fixed samples.
The XMAP215/Dis1 family is a highly conserved group of microtubule-associated proteins (MAPs) in eukaryotic organisms. These proteins are unique MAPs because they primarily interact with the growing-end (plus-end) of microtubules. This special property classifies this protein family as plus-end tracking proteins (+TIPs).

Don W. Cleveland is an American cancer biologist and neurobiologist.
Microtubule plus-end/positive-end tracking proteins or +TIPs are a type of microtubule associated protein (MAP) which accumulate at the plus ends of microtubules. +TIPs are arranged in diverse groups which are classified based on their structural components; however, all classifications are distinguished by their specific accumulation at the plus end of microtubules and their ability to maintain interactions between themselves and other +TIPs regardless of type. +TIPs can be either membrane bound or cytoplasmic, depending on the type of +TIPs. Most +TIPs track the ends of extending microtubules in a non-autonomous manner.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.
References
- ↑ Mohan, Renu; John, Annie (June 2015). "Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules: ROLE OF MAPs IN THE ACTIN-MICROTUBULE NETWORK". IUBMB Life. 67 (6): 395–403. doi: 10.1002/iub.1384 . PMID 26104829. S2CID 205968420.
- ^ Al-Bassam J, Ozer RS, Safer D, Halpain S, Milligan RA (June 2002). "MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments". J. Cell Biol. 157 (7): 1187–96. doi:10.1083/jcb.200201048. PMC 2173547 . PMID 12082079.
- ^ Childs, G. V. (2001) https://web.archive.org/web/20060424075523/http://www.cytochemistry.net/Cell-biology/microtubule_intro.htm, accessed 2/13/06.
- ^ Cooper, Geoffrey M., Hausman, Robert E. (2004) The Cell: A Molecular Approach. ASM Press, Washington D.C.
- ^ Drewes G, Ebneth A, Mandelkow EM (August 1998). "MAPs, MARKs and microtubule dynamics". Trends Biochem. Sci. 23 (8): 307–11. doi:10.1016/S0968-0004(98)01245-6. PMID 9757832.
- ^ Kar S, Fan J, Smith MJ, Goedert M, Amos LA (January 2003). "Repeat motifs of tau bind to the insides of microtubules in the absence of taxol". EMBO J. 22 (1): 70–7. doi:10.1093/emboj/cdg001. PMC 140040 . PMID 12505985.
- ^ Kinoshita K, Habermann B, Hyman AA (June 2002). "XMAP215: a key component of the dynamic microtubule cytoskeleton". Trends Cell Biol. 12 (6): 267–73. doi:10.1016/S0962-8924(02)02295-X. PMID 12074886.
- ^ Mandelkow E, Mandelkow EM (February 1995). "Microtubules and microtubule-associated proteins". Curr. Opin. Cell Biol. 7 (1): 72–81. doi:10.1016/0955-0674(95)80047-6. PMID 7755992.
- ^ Permana S, Hisanaga S, Nagatomo Y, Iida J, Hotani H, Itoh TJ (February 2005). "Truncation of the projection domain of MAP4 (microtubule-associated protein 4) leads to attenuation of microtubule dynamic instability". Cell Struct. Funct. 29 (5–6): 147–57. doi: 10.1247/csf.29.147 . PMID 15840946.
- ^ Santarella RA, Skiniotis G, Goldie KN, et al. (June 2004). "Surface-decoration of microtubules by human tau". J. Mol. Biol. 339 (3): 539–53. doi:10.1016/j.jmb.2004.04.008. PMID 15147841.
- Mohan, Renu; John, Annie (2015-06). "Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules: ROLE OF MAPs IN THE ACTIN_MICROTUBULE NETWORK" IUBMB Life. 67 (6): 395–403. doi:10.1002/iub.1384