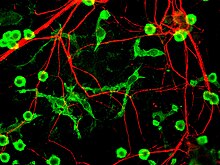
An axon, or nerve fiber, is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction has caused many inherited and acquired neurological disorders which can affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

Keratin is one of a family of structural fibrous proteins also known as scleroproteins. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, softer forms found in all vertebrates and harder, derived forms found only among sauropsids.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

The nuclear lamina is a dense fibrillar network inside the nucleus of eukaryote cells. It is composed of intermediate filaments and membrane associated proteins. Besides providing mechanical support, the nuclear lamina regulates important cellular events such as DNA replication and cell division. Additionally, it participates in chromatin organization and it anchors the nuclear pore complexes embedded in the nuclear envelope.
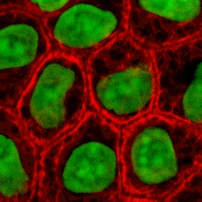
Cytokeratins are keratin proteins found in the intracytoplasmic cytoskeleton of epithelial tissue. They are an important component of intermediate filaments, which help cells resist mechanical stress. Expression of these cytokeratins within epithelial cells is largely specific to particular organs or tissues. Thus they are used clinically to identify the cell of origin of various human tumors.

Desmin is a protein that in humans is encoded by the DES gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.

Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks, but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the PRPH gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despite extensive research into how neurofilaments and peripherin contribute to ALS, their role in this disease is still unidentified.

Glial fibrillary acidic protein (GFAP) is a protein that is encoded by the GFAP gene in humans. It is a type III intermediate filament (IF) protein that is expressed by numerous cell types of the central nervous system (CNS), including astrocytes and ependymal cells during development. GFAP has also been found to be expressed in glomeruli and peritubular fibroblasts taken from rat kidneys, Leydig cells of the testis in both hamsters and humans, human keratinocytes, human osteocytes and chondrocytes and stellate cells of the pancreas and liver in rats.
Neurofilaments (NF) are classed as type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with microtubules (~25 nm) and microfilaments (7 nm), they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into six types based on their gene organization and protein structure. Types I and II are the keratins which are expressed in epithelia. Type III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Type IV consists of the neurofilament proteins L, M, H and internexin. Type V consists of the nuclear lamins, and type VI consists of the protein nestin. The type IV intermediate filament genes all share two unique introns not found in other intermediate filament gene sequences, suggesting a common evolutionary origin from one primitive type IV gene.

Spectrin is a cytoskeletal protein that lines the intracellular side of the plasma membrane in eukaryotic cells. Spectrin forms pentagonal or hexagonal arrangements, forming a scaffold and playing an important role in maintenance of plasma membrane integrity and cytoskeletal structure. The hexagonal arrangements are formed by tetramers of spectrin subunits associating with short actin filaments at either end of the tetramer. These short actin filaments act as junctional complexes allowing the formation of the hexagonal mesh. The protein is named spectrin since it was first isolated as a major protein component of human red blood cells which had been treated with mild detergents; the detergents lysed the cells and the hemoglobin and other cytoplasmic components were washed out. In the light microscope the basic shape of the red blood cell could still be seen as the spectrin-containing submembranous cytoskeleton preserved the shape of the cell in outline. This became known as a red blood cell "ghost" (spectre), and so the major protein of the ghost was named spectrin.
Crescentin is a protein which is a bacterial relative of the intermediate filaments found in eukaryotic cells. Just as tubulins and actins, the other major cytoskeletal proteins, have prokaryotic homologs in, respectively, the FtsZ and MreB proteins, intermediate filaments are linked to the crescentin protein. Some of its homologs are erroneously labelled Chromosome segregation protein ParA. This protein family is found in Caulobacter and Methylobacterium.

Nestin is a protein that in humans is encoded by the NES gene.
Tektins are cytoskeletal proteins found in cilia and flagella as structural components of outer doublet microtubules. They are also present in centrioles and basal bodies. They are polymeric in nature, and form filaments.

Neurofilament medium polypeptide (NF-M) is a protein that in humans is encoded by the NEFM gene.

Neurofilament light polypeptide, also known as neurofilament light chain, is a neurofilament protein that in humans is encoded by the NEFL gene. Neurofilament light chain is a biomarker that can be measured with immunoassays in cerebrospinal fluid and plasma and reflects axonal damage in a wide variety of neurological disorders. It is a useful marker for disease monitoring in amyotrophic lateral sclerosis, multiple sclerosis, Alzheimer's disease, and more recently Huntington's disease. It is also promising marker for follow-up of patients with brain tumors. Higher numbers have been associated with increased mortality.

Kinesin-like protein KIF1A, also known as axonal transporter of synaptic vesicles or microtubule-based motor KIF1A, is a protein that in humans is encoded by the KIF1A gene.
Fasciclin 2 is a 95 kilodalton cell membrane glycoprotein in the immunoglobulin (Ig) – related superfamily of cell adhesion molecules (CAMs). It was first identified in the developing grasshopper embryo, seen dynamically expressed on a subset of fasciculating axons in the central nervous system (CNS), functioning as a neuronal recognition molecule in the regulation of selective axon fasciculation. Subsequently, fasII was cloned and has mainly been studied in the fruit fly. Its extracellular structure consists of two Fibronectin type III domains and five Ig-like C2 domains, having structural homology to the neural cell adhesion molecule (NCAM) found in vertebrates. Alternative splicing of fasII gives rise to its expression in three major isoforms, including a membrane-associated form that is attached to the outer leaflet of the plasma membrane via a glycophosphatidylinositol linkage and two integral transmembrane forms. The larger transmembrane form has an amino acid motif contained in its cytoplasmic domain that is rich in proline, glutamic acid, serine and threonine residues. The fasciclin 1 (Fas1) and fasciclin 3 (Fas3) genes in Drosophila also code for cell adhesion proteins in the nervous system but do not show any structural or functional similarities with NCAM.
Alpha-keratin, or α-keratin, is a type of keratin found in vertebrates. This protein is the primary component in hairs, horns, mammalian claws, nails and the epidermis layer of the skin. α-keratin is a fibrous structural protein, meaning it is made up of amino acids that form a repeating secondary structure. The secondary structure of α-keratin is very similar to that of a traditional protein α-helix and forms a coiled coil. Due to its tightly wound structure, it can function as one of the strongest biological materials and has various functions in mammals, from predatory claws to hair for warmth. α-keratin is synthesized through protein biosynthesis, utilizing transcription and translation, but as the cell matures and is full of α-keratin, it dies, creating a strong non-vascular unit of keratinized tissue.

Neurotubules are microtubules found in neurons in nervous tissues. Along with neurofilaments and microfilaments, they form the cytoskeleton of neurons. Neurotubules are undivided hollow cylinders that are made up of tubulin protein polymers and arrays parallel to the plasma membrane in neurons. Neurotubules have an outer diameter of about 23 nm and an inner diameter, also known as the central core, of about 12 nm. The wall of the neurotubules is about 5 nm in width. There is a non-opaque clear zone surrounding the neurotubule and it is about 40 nm in diameter. Like microtubules, neurotubules are greatly dynamic and the length of them can be adjusted by polymerization and depolymerization of tubulin.