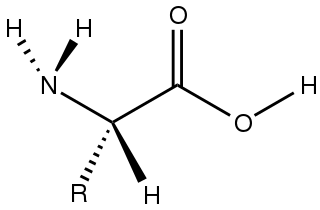
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life.
Chymotrypsin (EC 3.4.21.1, chymotrypsins A and B, alpha-chymar ophth, avazyme, chymar, chymotest, enzeon, quimar, quimotrase, alpha-chymar, alpha-chymotrypsin A, alpha-chymotrypsin) is a digestive enzyme component of pancreatic juice acting in the duodenum, where it performs proteolysis, the breakdown of proteins and polypeptides. Chymotrypsin preferentially cleaves peptide amide bonds where the side chain of the amino acid N-terminal to the scissile amide bond (the P1 position) is a large hydrophobic amino acid (tyrosine, tryptophan, and phenylalanine). These amino acids contain an aromatic ring in their side chain that fits into a hydrophobic pocket (the S1 position) of the enzyme. It is activated in the presence of trypsin. The hydrophobic and shape complementarity between the peptide substrate P1 side chain and the enzyme S1 binding cavity accounts for the substrate specificity of this enzyme. Chymotrypsin also hydrolyzes other amide bonds in peptides at slower rates, particularly those containing leucine at the P1 position.

In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 of one alpha-amino acid and N2 of another, along a peptide or protein chain.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

Cysteine is a semiessential proteinogenic amino acid with the formula HOOC−CH(−NH2)−CH2−SH. The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but interestingly, both D and L-cysteine are found in nature with D-cysteine having been found in developing brain.

Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC.

Flavins refers generally to the class of organic compounds containing the tricyclic heterocycle isoalloxazine or its isomer alloxazine, and derivatives thereof. The biochemical source of flavin is the yellow B vitamin riboflavin. The flavin moiety is often attached with an adenosine diphosphate to form flavin adenine dinucleotide (FAD), and, in other circumstances, is found as flavin mononucleotide, a phosphorylated form of riboflavin. It is in one or the other of these forms that flavin is present as a prosthetic group in flavoproteins. Despite the similar names, flavins are chemically and biologically distinct from the flavanoids, and the flavonols.

Post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes translating mRNA into polypeptide chains, which may then change to form the mature protein product. PTMs are important components in cell signalling, as for example when prohormones are converted to hormones.

Melamine is an organic compound with the formula C3H6N6. This white solid is a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 67% nitrogen by mass, and its derivatives have fire-retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high pressure decorative laminates such as Formica, melamine dinnerware including cooking utensils, plates, plastic products, laminate flooring, and dry erase boards. Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser.

The Codex Alimentarius is a collection of internationally recognized standards, codes of practice, guidelines, and other recommendations published by the Food and Agriculture Organization of the United Nations relating to food, food production, food labeling, and food safety.

In organic chemistry, peptide synthesis is the production of peptides, compounds where multiple amino acids are linked via amide bonds, also known as peptide bonds. Peptides are chemically synthesized by the condensation reaction of the carboxyl group of one amino acid to the amino group of another. Protecting group strategies are usually necessary to prevent undesirable side reactions with the various amino acid side chains. Chemical peptide synthesis most commonly starts at the carboxyl end of the peptide (C-terminus), and proceeds toward the amino-terminus (N-terminus). Protein biosynthesis in living organisms occurs in the opposite direction.

Papain, also known as papaya proteinase I, is a cysteine protease enzyme present in papaya and mountain papaya. It is the namesake member of the papain-like protease family.
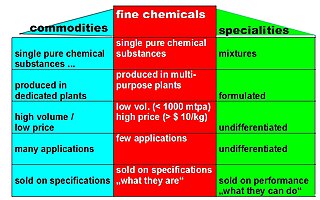
In chemistry, fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg. The class of fine chemicals is subdivided either on the basis of the added value, or the type of business transaction, namely standard or exclusive products.
Neurokinin 1 (NK1) antagonists (-pitants) are a novel class of medications that possesses unique antidepressant, anxiolytic, and antiemetic properties. NK-1 antagonists boost the efficacy of 5-HT3 antagonists to prevent nausea and vomiting. The discovery of neurokinin 1 (NK1) receptor antagonists was a turning point in the prevention of nausea and vomiting associated with cancer chemotherapy.

Ovotransferrin (conalbumin) is a glycoprotein of egg white albumen. Egg white albumen is composed of multiple proteins, of which ovotransferrin is the most heat reliable. It has a molecular weight of 76,000 daltons and contains about 700 amino acids. Ovotransferrin makes up approximately 13% of egg albumen. As a member of the transferrin and metalloproteinase family, ovotransferrin has been found to possess antibacterial and antioxydant and immunomodulatory properties, arising primarily through its iron (Fe3+) binding capacity by locking away a key biochemical component necessary for micro-organismal survival. Bacteria starved of iron are rendered incapable of moving, making ovotransferrin a potent bacteriostatic.
Carboxypeptidase A usually refers to the pancreatic exopeptidase that hydrolyzes peptide bonds of C-terminal residues with aromatic or aliphatic side-chains. Most scientists in the field now refer to this enzyme as CPA1, and to a related pancreatic carboxypeptidase as CPA2.
In China, the adulteration and contamination of several food and feed ingredients with inexpensive melamine and other compounds, such as cyanuric acid, ammeline and ammelide, are common practice. These adulterants can be used to inflate the apparent protein content of products, so that inexpensive ingredients can pass for more expensive, concentrated proteins. Melamine by itself has not been thought to be very toxic to animals or humans except possibly in very high concentrations, but the combination of melamine and cyanuric acid has been implicated in kidney failure. Reports that cyanuric acid may be an independently and potentially widely used adulterant in China have heightened concerns for both animal and human health.

A food contaminant is a harmful chemical or microorganism present in food, which can cause illness to the consumer.
Dietary exposure assessments in the United States involve the evaluation of dietary consumption and chemical residue data while taking into consideration additional factors that may affect a specified population of interest or sensitive population. The process of conducting a dietary exposure assessment involves the determination of the chemical residues on a particular food or foods and the calculation of the dietary exposure to these chemicals based on consumption data for the specified food or foods. A dietary exposure assessment allows a comparison to a relevant health standard such as the acceptable daily intake(ADI), the acute reference dose.
















