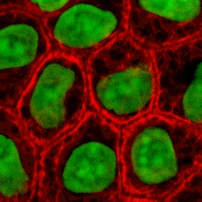
Keratin is one of a family of structural fibrous proteins also known as scleroproteins. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, softer forms found in all vertebrates and harder, derived forms found only among sauropsids.

The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth or disassembly depending on the cell's requirements.

Intermediate filaments (IFs) are cytoskeletal structural components found in the cells of vertebrates, and many invertebrates. Homologues of the IF protein have been noted in an invertebrate, the cephalochordate Branchiostoma.

Keratin, type II cytoskeletal 7 also known as cytokeratin-7 (CK-7) or keratin-7 (K7) or sarcolectin (SCL) is a protein that in humans is encoded by the KRT7 gene. Keratin 7 is a type II keratin. It is specifically expressed in the simple epithelia lining the cavities of the internal organs and in the gland ducts and blood vessels.

Keratin 6A is one of the 27 different type II keratins expressed in humans. Keratin 6A was the first type II keratin sequence determined. Analysis of the sequence of this keratin together with that of the first type I keratin led to the discovery of the four helical domains in the central rod of keratins. In humans Keratin 6A is encoded by the KRT6A gene.
Type II keratins constitutes the Type II intermediate filaments (IFs) of the intracytoplasmatic cytoskeleton, which is present in all mammalian epithelial cells. The type 2 cytokeratins consist of basic or neutral, high molecular weight proteins which in vivo are arranged in pairs of heterotypic Type I and Type II keratin chains, coexpressed during differentiation of simple and stratified epithelial tissues. It has been seen that Type II Keratins are developed before Type 1 keratins during human embryonic development.
Type I keratins are cytokeratins that constitute the Type I intermediate filaments (IFs) of the intracytoplasmatic cytoskeleton, which is present in all mammalian epithelial cells. Most of the type I keratins consist of acidic, low molecular weight proteins which in vivo are arranged in pairs of heterotypic Type I and Type II keratin chains, coexpressed during differentiation of simple and stratified epithelial tissues.

Keratin, type I cytoskeletal 10 also known as cytokeratin-10 (CK-10) or keratin-10 (K10) is a protein that in humans is encoded by the KRT10 gene. Keratin 10 is a type I keratin.

Keratin, type I cytoskeletal 19 also known as cytokeratin-19 (CK-19) or keratin-19 (K19) is a 40 kDa protein that in humans is encoded by the KRT19 gene. Keratin 19 is a type I keratin.

Keratin 18 is a type I cytokeratin. It is, together with its filament partner keratin 8, perhaps the most commonly found products of the intermediate filament gene family. They are expressed in single layer epithelial tissues of the body. Mutations in this gene have been linked to cryptogenic cirrhosis. Two transcript variants encoding the same protein have been found for this gene.
Hair keratin is a type of keratin found in hair and the nails.
Internexin, alpha-internexin, is a Class IV intermediate filament approximately 66 KDa. The protein was originally purified from rat optic nerve and spinal cord. The protein copurifies with other neurofilament subunits, as it was originally discovered, however in some mature neurons it can be the only neurofilament expressed. The protein is present in developing neuroblasts and in the central nervous system of adults. The protein is a major component of the intermediate filament network in small interneurons and cerebellar granule cells, where it is present in the parallel fibers.

Vimentin is a structural protein that in humans is encoded by the VIM gene. Its name comes from the Latin vimentum which refers to an array of flexible rods.
Neurofilaments (NF) are classed as type IV intermediate filaments found in the cytoplasm of neurons. They are protein polymers measuring 10 nm in diameter and many micrometers in length. Together with microtubules (~25 nm) and microfilaments (7 nm), they form the neuronal cytoskeleton. They are believed to function primarily to provide structural support for axons and to regulate axon diameter, which influences nerve conduction velocity. The proteins that form neurofilaments are members of the intermediate filament protein family, which is divided into six types based on their gene organization and protein structure. Types I and II are the keratins which are expressed in epithelia. Type III contains the proteins vimentin, desmin, peripherin and glial fibrillary acidic protein (GFAP). Type IV consists of the neurofilament proteins NF-L, NF-M, NF-H and α-internexin. Type V consists of the nuclear lamins, and type VI consists of the protein nestin. The type IV intermediate filament genes all share two unique introns not found in other intermediate filament gene sequences, suggesting a common evolutionary origin from one primitive type IV gene.
Fimbrin also known as is plastin 1 is a protein that in humans is encoded by the PLS1 gene. Fimbrin is an actin cross-linking protein important in the formation of filopodia.
Crescentin is a protein which is a bacterial relative of the intermediate filaments found in eukaryotic cells. Just as tubulins and actins, the other major cytoskeletal proteins, have prokaryotic homologs in, respectively, the FtsZ and MreB proteins, intermediate filaments are linked to the crescentin protein. Some of its homologs are erroneously labelled Chromosome segregation protein ParA. This protein family is found in Caulobacter and Methylobacterium.

Villin-1 is a 92.5 kDa tissue-specific actin-binding protein associated with the actin core bundle of the brush border. Villin-1 is encoded by the VIL1 gene. Villin-1 contains multiple gelsolin-like domains capped by a small "headpiece" at the C-terminus consisting of a fast and independently folding three-helix bundle that is stabilized by hydrophobic interactions. The headpiece domain is a commonly studied protein in molecular dynamics due to its small size and fast folding kinetics and short primary sequence.

Keratin, type II cytoskeletal 8 also known as cytokeratin-8 (CK-8) or keratin-8 (K8) is a keratin protein that is encoded in humans by the KRT8 gene. It is often paired with keratin 18.

Keratin 5, also known as KRT5, K5, or CK5, is a protein that is encoded in humans by the KRT5 gene. It dimerizes with keratin 14 and forms the intermediate filaments (IF) that make up the cytoskeleton of basal epithelial cells. This protein is involved in several diseases including epidermolysis bullosa simplex and breast and lung cancers.
Alpha-keratin, or α-keratin, is a type of keratin found in mammalian vertebrates. This protein is the primary component in hairs, horns, claws, nails and the epidermis layer of the skin. α-keratin is a fibrous structural protein, meaning it is made up of amino acids that form a repeating secondary structure. The secondary structure of α-keratin is very similar to that of a traditional protein α-helix and forms a coiled coil. Due to its tightly wound structure, it can function as one of the strongest biological materials and has various functions in mammals, from predatory claws to hair for warmth. α-keratin is synthesized through protein biosynthesis, utilizing transcription and translation, but as the cell matures and is full of α-keratin, it dies, creating a strong non-vascular unit of keratinized tissue.