A phospholipase is an enzyme that hydrolyzes phospholipids into fatty acids and other lipophilic substances. Acids trigger the release of bound calcium from cellular stores and the consequent increase in free cytosolic Ca2+, an essential step in calcium signaling to regulate intracellular processes. There are four major classes, termed A, B, C, and D, which are distinguished by the type of reaction which they catalyze:

Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the Neo-Latin word arachis (peanut), but peanut oil does not contain any arachidonic acid.

The enzyme phospholipase A2 (EC 3.1.1.4, PLA2, systematic name phosphatidylcholine 2-acylhydrolase) catalyse the cleavage of fatty acids in position 2 of phospholipids, hydrolyzing the bond between the second fatty acid “tail” and the glycerol molecule:
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

Infantile neuroaxonal dystrophy is a rare pervasive developmental disorder that primarily affects the nervous system. Individuals with infantile neuroaxonal dystrophy typically do not have any symptoms at birth, but between the ages of about 6 and 18 months they begin to experience delays in acquiring new motor and intellectual skills, such as crawling or beginning to speak. Eventually they lose previously acquired skills.

Cyclooxygenase 1 (COX-1), also known as prostaglandin-endoperoxide synthase 1, is an enzyme that in humans is encoded by the PTGS1 gene. In humans it is one of two cyclooxygenases.
In enzymology, a ceramide kinase, also abbreviated as CERK, is an enzyme that catalyzes the chemical reaction:

Cytosolic phospholipase A2 is an enzyme that in humans is encoded by the PLA2G4A gene.

Phospholipase C (PLC) is a class of membrane-associated enzymes that cleave phospholipids just before the phosphate group (see figure). It is most commonly taken to be synonymous with the human forms of this enzyme, which play an important role in eukaryotic cell physiology, in particular signal transduction pathways. Phospholipase C's role in signal transduction is its cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which serve as second messengers. Activators of each PLC vary, but typically include heterotrimeric G protein subunits, protein tyrosine kinases, small G proteins, Ca2+, and phospholipids.

Calcium-dependent phospholipase A2 is an enzyme that in humans is encoded by the PLA2G5 gene.

Peroxiredoxin-6 is a protein that in humans is encoded by the PRDX6 gene. It is a member of the peroxiredoxin family of antioxidant enzymes.

Cytosolic phospholipase A2 gamma is an enzyme that in humans is encoded by the PLA2G4C gene.

Group 10 secretory phospholipase A2 is an enzyme that in humans is encoded by the PLA2G10 gene.

Group XVI phospholipase A2 also commonly known as adipocyte phospholipase A2 (AdPLA) is an enzyme that in humans is encoded by the PLA2G16 gene. This enzyme has also been identified as PLA2G16, HRASLS3, HREV107, HREV107-3, MGC118754 or H-REV107-1 from studies on class II tumor suppression but not on its enzymatic properties. AdPLA is encoded by a 1.3 kilobase AdPLA messenger RNA and is an 18 kDa protein. It belongs to a superfamily of phospholipase A2 (PLA2) enzymes and is found primarily in adipose tissue. AdPLA regulates adipocyte lipolysis and release of fatty acids through a G-protein coupled pathway involving prostaglandin and EP3. It has also been reported to play a crucial role in the development of obesity in mouse models.

Patatin-like phospholipase domain-containing protein 3 (PNPLA3) also known as adiponutrin (ADPN), acylglycerol O-acyltransferase or calcium-independent phospholipase A2-epsilon (iPLA2-epsilon) is an enzyme that in humans is encoded by the PNPLA3 gene.

Lipoprotein-associated phospholipase A2 (Lp-PLA2) also known as platelet-activating factor acetylhydrolase (PAF-AH) is a phospholipase A2 enzyme that in humans is encoded by the PLA2G7 gene. Lp-PLA2 is a 45-kDa protein of 441 amino acids. It is one of several PAF acetylhydrolases.

CYP2U1 is a protein that in humans is encoded by the CYP2U1 gene

Neuropathy target esterase, also known as patatin-like phospholipase domain-containing protein 6 (PNPLA6), is an esterase enzyme that in humans is encoded by the PNPLA6 gene.
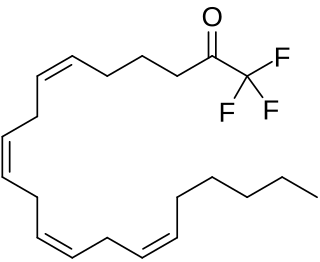
Arachidonyl trifluoromethyl ketone (ATK) is an analog of arachidonic acid. that inhibits some isoforms of the enzyme phospholipase A2. Specifically it inhibits the 85 kDa cystolic PLA2 (cPLA2).
Crotoxin (CTX) is the main toxic compound in the snake venom of the South American rattlesnake, Crotalus durissus terrificus. Crotoxin is a heterodimeric beta-neurotoxin, composed of an acidic, non-toxic and non-enzymatic subunit (CA), and a basic, weakly toxic, phospholipase A2 protein (CB). This neurotoxin causes paralysis by both pre- and postsynaptic blocking of acetylcholine signalling.