
A leaf spot is a limited, discoloured, diseased area of a leaf that is caused by fungal, bacterial or viral plant diseases, or by injuries from nematodes, insects, environmental factors, toxicity or herbicides. These discoloured spots or lesions often have a centre of necrosis. Symptoms can overlap across causal agents, however differing signs and symptoms of certain pathogens can lead to the diagnosis of the type of leaf spot disease. Prolonged wet and humid conditions promote leaf spot disease and most pathogens are spread by wind, splashing rain or irrigation that carry the disease to other leaves.

Tomato bushy stunt virus (TBSV) is a virus of the tombusvirus family. It was first reported in tomatoes in 1935 and primarily affects vegetable crops, though it is not generally considered an economically significant plant pathogen. Depending upon the host, TBSV causes stunting of growth, leaf mottling, and deformed or absent fruit. The virus is likely to be soil-borne in the natural setting, but can also be transmitted mechanically, for example through contaminated cutting tools. TBSV has been used as a model system in virology research on the life cycle of plant viruses, particularly in experimental infections of the model host plant Nicotiana benthamiana.
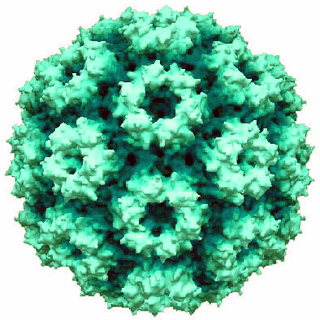
Cowpea chlorotic mottle virus, known by the abbreviation CCMV, is a virus that specifically infects the cowpea plant, or black-eyed pea. The leaves of infected plants develop yellow spots, hence the name "chlorotic". Similar to its "brother" virus, Cowpea mosaic virus (CPMV), CCMV is produced in high yield in plants. In the natural host, viral particles can be produced at 1–2 mg per gram of infected leaf tissue. Belonging to the bromovirus genus, cowpea chlorotic mottle virus (CCMV) is a small spherical plant virus. Other members of this genus include the brome mosaic virus (BMV) and the broad bean mottle virus (BBMV).

Alfalfa mosaic virus (AMV), also known as Lucerne mosaic virus or Potato calico virus, is a worldwide distributed phytopathogen that can lead to necrosis and yellow mosaics on a large variety of plant species, including commercially important crops. It is the only Alfamovirus of the family Bromoviridae. In 1931 Weimer J.L. was the first to report AMV in alfalfa. Transmission of the virus occurs mainly by some aphids, by seeds or by pollen to the seed.

Abacarus hystrix, the cereal rust mite or grain rust mite, belongs to the family Eriophyidae. They are extremely small with adults measuring up to 1 millimetre in length and only have four legs at the front of the body. Viewing by the human eye requires a 10 – 20X lens. The adult mites are usually yellow but also have been seen to be white or orange. The cereal rust mite was first found on Elymus repens, a very common perennial grass species. It has now been found on more than 60 grass species including oats, barley, wheat and ryegrass, found in Europe, North America, South Africa and Australia. Mites migrate primarily through wind movement and are usually found on the highest basal sections of the top two leaf blades. Abacarus hystrix produces up to twenty overlapping generations per year in South Australian perennial pastures, indicating that the species breeds quite rapidly. It has been noted that the cereal rust mite can cause losses in yield of up to 30-70%.

Apple mosaic virus (ApMV) is a plant pathogenic virus of the family Bromoviridae. It is named after its symptoms that were first present on apples. ApMV is a positive sense RNA based virus. The disease itself has several synonyms including Mild Apple Mosaic Virus, Hop Virus, Rose Mosaic Virus, and European Plum Line Patten Virus. It causes a severe yield reduction and decreased life-expectancy of fruit trees.
Barley stripe mosaic virus (BSMV), of genus Hordevirus, is an RNA viral plant pathogen whose main hosts are barley and wheat. The common symptoms for BSMV are yellow streaks or spots, mosaic, leaves and stunted growth. It is spread primarily through infected seed and can be spread through mechanical transfer of an infected and uninfected host. Plants infected with BSMV are more symptomatic in warmer temperatures. Resistant hosts and sterilization of equipment are the best ways to control the spread of the pathogen. BSMV has been known to reduce the yields of barley by up to 25%, but is not a major problem because of resistant varieties of barley.

Cucumber mosaic virus (CMV) is a plant pathogenic virus in the family Bromoviridae. This virus has a worldwide distribution and a very wide host range, having the reputation of the widest host range of any known plant virus. It can be transmitted from plant to plant both mechanically by sap and by aphids in a stylet-borne fashion. It can also be transmitted in seeds and by the parasitic weeds, Cuscuta sp. (dodder).

Maize dwarf mosaic virus (MDMV) is a pathogenic plant virus of the family Potyviridae. Depending on the corn plant’s growth stage, the virus can have severe implications to the corn plant’s development which can also result in economic consequences to the producer of the crop.

Pepper mild mottle virus (PMMoV) is a plant pathogenic virus that occurs worldwide on species of field grown bell, hot and ornamental pepper species. It is caused by members of the plant virus genus Tobamovirus- otherwise known as the tobacco mosaic virus family. Tobamovirus are viruses that contain positive sense RNA genomes that infect plants. Symptoms of the disease vary depending on the cultivar. Typical symptoms include the chlorosis of leaves, stunting, and distorted and lumpy fruiting structures. The virus is spread by mechanical transmission and infected seeds. Avoidance is the best means of controlling the disease because once a plant is infected it cannot be treated. Only seeds that have been tested and treated for the pathogen should be planted.
Potato virus Y (PVY) is a plant pathogenic virus of the family Potyviridae, and one of the most important plant viruses affecting potato production.
Soil-borne wheat mosaic virus is a rod-shaped plant pathogen that can cause severe stunting and mosaic in susceptible wheat, barley and rye cultivars. The disease has often been misdiagnosed as a nutritional problem, but this has actually allowed in part for the fortuitous visual selection by breeding programs of resistant genotypes. Soil-borne wheat mosaic virus is part of the genus Furovirus. Members of this genus are characterized by rigid rod-shaped particles and positive sense RNA genomes consisting of two molecules that are packaged into separate particles that code for either replication, mobility, structure or defense against the host. The virus is spread by a fungal-like protist, Polymyxa graminis, whose asexual secondary and sexual primary cycles help the virus spread. The disease produces secondary symptoms from the root cell infection. The disease is a serious contributor to loss in crop yield.

Panicum mosaic satellite virus (SPMV) is a plant satellite virus in genus Papanivirus, which is a member of realm Riboviria without assigned family or order. It only infects grasses which are infected by Panicum mosaic virus. One study found that 72% of Stenotaphrum secundatum infected with panicum mosaic virus was also infected with SPMV. In addition to SPMV, many plants infected with panicum mosaic virus are also infected with satellite RNAs.

Soybean mosaic virus (SMV) is a member of the plant virus genus Potyvirus. It infects mainly plants belonging to the family Fabaceae but has also been found infecting other economically important crops. SMV is the cause of soybean mosaic disease that occurs in all the soybean productions areas of the world. Soybean is one of the most important sources of edible oil and proteins and pathogenic infections are responsible for annual yield losses of about $4 billion in the United States. Among these pathogens, SMV is the most important and prevalent viral pathogen in soybean production worldwide. It causes yield reductions of about 8% to 35% but losses as high as 94% have been reported.

Watermelon mosaic virus (WMV) also known as Marrow mosaic virus, Melon mosaic virus, and until recently Watermelon mosaic virus type 2 (WMV-2), is a plant pathogenic virus that causes viral infection in many different plants. The virus itself is referred to as Watermelon Mosaic Virus II or WMV-2 and is an isolate of the U.S. WMV-2 is a ssRNA positive strand virus that is part of the Potyviridae or Potyvirus clade. Like all RNA viruses, it contains a protein capsid which protects the inner viral RNA. First described on squash in Florida, WMV arose from a unique recombination of genetic material contributed by Soybean mosaic virus (SMV) and Bean common mosaic virus (BCMV) along with Peanut Stripe virus (PSV).
Bacterial wilt of turfgrass is the only known bacterial disease of turf. The causal agent is the Gram negative bacterium Xanthomonas campestris pv. graminis. The first case of bacterial wilt of turf was reported in a cultivar of creeping bentgrass known as Toronto or C-15, which is found throughout the midwestern United States. Until the causal agent was identified in 1984, the disease was referred to simply as C-15 decline. This disease is almost exclusively found on putting greens at golf courses where extensive mowing creates wounds in the grass which the pathogen uses in order to enter the host and cause disease.
Mastrevirus is a genus of ssDNA viruses, in the family Geminiviridae. Mostly monocotyledonous plants serve as natural hosts. They are vectored by planthoppers. There are 45 species in this genus. Diseases associated with this genus include: maize streak virus: maize streak disease (MSD).
Cocoa necrosis virus (CoNV) is a plant pathogenic virus of the genus nepovirus that infects Theobroma cacao en natura causing cacao necrosis disease. CoNV is considered synonymous with Strain S of cacao swollen shoot virus. Unlike Cacao swollen shoot virus, it is not transmitted by mealybugs nor vectored by aphids, beetles, or leafhoppers that also commonly infest cacao. It is serologically, distantly related to Tomato black ring virus and very distantly related to Grapevine chrome mosaic virus.
The cardamom mosaic virus (CdMV) is a mosaic virus that affects the production of green cardamom (E. cardamomum). It is a member of the genus Macluravirus (recognized under the family Potyviridae by ICTV in 1988), and is transmitted through aphids (P.caladii) and infected rhizomes, the former in a non-persistent manner.

Viral diseases of potato are a group of diseases caused by different types of viruses that affect potato crops worldwide and, although they do not affect human or animal health since they are viruses that only infect vegetables, they are a source of great economic losses annually. About 28 viruses have been reported infecting potato crops. However, potato virus X (PVX), potato virus Y (PVY), and potato leafroll virus (PLRV) are the most important viruses worldwide. Some others are of economic importance only in some regions. Such is the case of potato virus M (PVM) in some Asian and European countries.












