
Perfluorooctanoic acid is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a material feedstock. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, n-heptyl "tail group" and a carboxylate "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic.

Perfluorooctanesulfonic acid (PFOS) is a chemical compound having an eight-carbon fluorocarbon chain and a sulfonic acid functional group and thus a perfluorosulfonic acid. It is an anthropogenic (man-made) fluorosurfactant, now regarded as a global pollutant. PFOS was the key ingredient in Scotchgard, a fabric protector made by 3M, and related stain repellents. The acronym "PFOS" refers to the parent sulfonic acid and to various salts of perfluorooctanesulfonate. These are all colorless or white, water-soluble solids. Although of low acute toxicity, PFOS has attracted much attention for its pervasiveness and environmental impact. It was added to Annex B of the Stockholm Convention on Persistent Organic Pollutants in May 2009.

Stockholm Convention on Persistent Organic Pollutants is an international environmental treaty, signed on 22 May 2001 in Stockholm and effective from 17 May 2004, that aims to eliminate or restrict the production and use of persistent organic pollutants (POPs).

Persistent organic pollutants (POPs) are organic compounds that are resistant to degradation through chemical, biological, and photolytic processes. They are toxic and adversely affect human health and the environment around the world. Because they can be transported by wind and water, most POPs generated in one country can and do affect people and wildlife far from where they are used and released.
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Scotchgard is a 3M brand of products, a stain and durable water repellent applied to fabric, furniture, and carpets to protect them from stains. Scotchgard products typically rely on organofluorine chemicals as the main active ingredient along with petroleum distillate solvents.
Perfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also an environmental contaminant found in people and wildlife along with PFOS and PFOA.

Per- and polyfluoroalkyl substances are a group of synthetic organofluorine chemical compounds that have multiple fluorine atoms attached to an alkyl chain. The PubChem database lists more than 6 million unique compounds in this group. PFASs started being used in the mid-20th century to make fluoropolymer coatings and products that resist heat, oil, stains, grease, and water. They are used in a variety of products including waterproof clothing, furniture, adhesives, food packaging, heat-resistant non-stick cooking surfaces, and the insulation of electrical wire. They have played a key economic role for companies such as DuPont, 3M, and W. L. Gore & Associates that use them to produce widely known materials such as Teflon or Gore-Tex.
Fluorotelomer alcohols, or FTOHs, are fluorotelomers with an alcohol functional group. They are volatile precursors to perfluorinated carboxylic acids, such as PFOA and PFNA, and other compounds.

Perfluorobutanesulfonic acid (PFBS) is a PFAS chemical compound having a four-carbon fluorocarbon chain and a sulfonic acid functional group. It is stable and unreactive because of the strength of carbon–fluorine bonds. It can occur in the form of a colorless liquid or a corrosive solid. Its conjugate base is perfluorobutanesulfonate which functions as the hydrophobe in fluorosurfactants.
Fluorotelomers are fluorocarbon-based oligomers, or telomers, synthesized by telomerization. Some fluorotelomers and fluorotelomer-based compounds are a source of environmentally persistent perfluorinated carboxylic acids such as PFOA and PFNA, while others are under extended investigation.
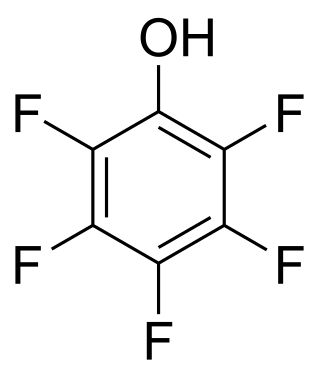
A perfluorinated compound (PFC) or perfluoro compound is an organofluorine compound lacking C-H bonds. Many perfluorinated compounds have properties that are quite different from their C-H containing analogues. Common functional groups in PFCs are OH, CO2H, chlorine, O, and SO3H. Electrofluorination is the predominant method for PFC production. Due to their chemical stability, some of these perfluorinated compounds bioaccumulate.

Perfluorooctanesulfonamide (PFOSA) is a synthetic organofluorine compound. It is a fluorocarbon derivative and a perfluorinated compound, having an eight-carbon chain and a terminal sulfonamide functional group. PFOSA, a persistent organic pollutant, was an ingredient in 3M's former Scotchgard formulation from 1956 until 2003, and the compound was used to repel grease and water in food packaging along with other consumer applications. It breaks down to form perfluorooctane sulfonate (PFOS). The perfluorooctanesulfonyl fluoride-based chemistry that was used to make sulfonamides like PFOSA was phased out by 3M in the United States (US) during 2000–2002 but it has grown in China by other producers.
The Global Monitoring Plan (GMP) under the Stockholm Convention on Persistent Organic Pollutants is a programme that enables collection of comparable monitoring data from all regions of the world to assess the effectiveness of the Stockholm Convention in minimizing human and environmental exposure to persistent organic pollutants (POPs). To know whether the levels of POPs are increasing or decreasing over time, information on environmental and human exposure levels of these chemicals should enable detection of trends. GMP looks at background levels of POPs at locations not influenced by local sources, such as ‘hot spots’. For human sampling, the focus is on the general population rather than on individuals who may have suffered high exposure to POPs.

Water contamination in Lawrence and Morgan Counties, Alabama, revolves around the presence of perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in the water supply. After the US Environmental Protection Agency (EPA) released new health advisories in March 2016, there was concern over health risks of the levels of PFOA and PFOS present. The responses of different government officials, agencies, and companies raise questions as to whether or not there was any environmental injustice involved.
This timeline of events related to per- and polyfluoroalkyl substances (PFASs) includes events related to the discovery, development, manufacture, marketing, uses, concerns, litigation, regulation, and legislation, involving the human-made PFASs. The timeline focuses on some perfluorinated compounds, particularly perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) and on the companies that manufactured and marketed them, mainly DuPont and 3M. An example of PFAS is the fluorinated polymer polytetrafluoroethylene (PTFE), which has been produced and marketed by DuPont under its trademark Teflon. GenX chemicals and perfluorobutanesulfonic acid (PFBS) are organofluorine chemicals used as a replacement for PFOA and PFOS.

Perfluorosulfonic acids (PFSAs) are chemical compounds of the formula CnF(2n+1)SO3H and thus belong to the family of perfluorinated and polyfluorinated alkyl compounds (PFASs). The simplest example of a perfluorosulfonic acid is the trifluoromethanesulfonic acid. Perfluorosulfonic acids with six or more perfluorinated carbon atoms, i.e. from perfluorohexanesulfonic acid onwards, are referred to as long-chain.
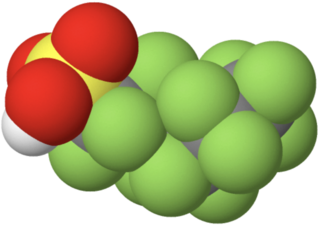
Perfluorohexanesulfonic acid (PFHxS) is a synthetic chemical compound. It is one of many compounds collectively known as per- and polyfluoroalkyl substances (PFASs). It is an anionic fluorosurfactant and a persistent organic pollutant with bioaccumulative properties. Although the use of products containing PFHxS and other PFASs have been banned or are being phased out in many jurisdictions, it remains ubiquitous in many environments and within the general population, and is one of the most commonly detected PFASs.
Sulfluramid (N-EtFOSA) is a chemical compound from the group of sulfonic acid amides and per- and polyfluoroalkyl substances (PFASs) that is effective as an insecticide.












