Phaseolus is a genus of herbaceous to woody annual and perennial vines in the family Fabaceae containing about 70 plant species, all native to the Americas, primarily Mesoamerica.

Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined, not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized de novo by plants that produce the compounds rapidly at sites of pathogen infection. In general phytoalexins are broad spectrum inhibitors; they are chemically diverse, and different chemical classes of compounds are characteristic of particular plant taxa. Phytoalexins tend to fall into several chemical classes, including terpenoids, glycosteroids and alkaloids, however the term applies to any phytochemicals that are induced by microbial infection.
Respiratory burst is the rapid release of the reactive oxygen species (ROS), superoxide anion and hydrogen peroxide, from different cell types.
Colin Louis Avern Leakey was a leading plant scientist in the United Kingdom, a Fellow of King's College, Cambridge and of the Institute of Biology, and a world authority on beans.
Alternaria carthami is a necrotrophic plant pathogen of safflower in the order Pleosporales and family Pleosporaceae. This fungus, first isolated in India, has spread globally and can have devastating effects on safflower yield, and resultant oilseed production. A. carthami is known to be seed-borne and appears as irregular brown lesions on safflower leaves and stems.
Mycocentrospora acerina is a deuteromycete fungus that is a plant pathogen.

Pterocarpans are derivatives of isoflavonoids found in the family Fabaceae. It is a group of compounds which can be described as benzo-pyrano-furano-benzenes which can be formed by coupling of the B ring to the 4-one position.
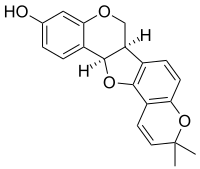
The molecular formula C20H18O4 may refer to:
Phaseolin is the main reserve globulin in seeds of the French bean. It was named and first isolated and characterized by Thomas Burr Osborne in 1894.
Erythrina subumbrans is a flowering plant species in the genus Erythrina.

6-Methoxymellein is a dihydroisocoumarin, a phenolic compound found in carrots and carrot purées. It is responsible for bitterness in carrots. It is a phytoalexin, induced in carrot slices by UV-C, that allows resistance to Botrytis cinerea and other microorganisms.
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.

Falcarindiol is a polyyne found in carrot roots which has antifungal activity. Falcarindiol is the main compound responsible for bitterness in carrots. Falcarindiol and other falcarindiol-type polyacetylenes are also found in many other plants of the family Apiaceae, including some commonly used seasonings such as dill and parsley. A variety of bioactivities have been reported so far for falcaridiol and the falcarindiol-type polyacetylenes, and because of potential health-promoting metabolic effects these compounds are studied as potential nutraceuticals. It is the most-active among several polyynes with potential anticancer activity found in Devil's Club, a medicinal plant used by many indigenous peoples in Alaska and the Pacific Northwest.
Pararhizobium giardinii is a Gram negative root nodule bacteria. It forms nitrogen-fixing root nodules on legumes, being first isolated from those of Phaseolus vulgaris.

Pisatin (3-hydroxy-7-methoxy-4′,5′-methylenedioxy-chromanocoumarane) is the major phytoalexin made by the pea plant Pisum sativum. It was the first phytoalexin to be purified and chemically identified. The molecular formula is C17H14O6.
Paraheliotropism refers to the phenomenon in which plants orient their leaves parallel to incoming rays of light, usually as a means of minimizing excess light absorption. Excess light absorption can cause a variety of physiological problems for plants, including overheating, dehydration, loss of turgor, photoinhibition, photo-oxidation, and photorespiration, so paraheliotropism can be viewed as an advantageous behavior in high light environments. Not all plants exhibit this behavior, but it has developed in multiple lineages.
Soon Jai Park, Ph.D. (1937–2018) was a Canadian federal research scientist at Agriculture and Agri-Food Canada in Harrow, Ontario. He was internationally known for his dry bean breeding program that expanded bean production in Canada – taking beans that are usually grown in Africa, India, Korea, Japan and Brazil, and breeding them so they could thrive in Ontario and Western Canada to give farmers new crops to grow. He developed more than 28 bean varieties during his 25 year career
Platypria (Platypria) hystrix, is a species of leaf beetle found in India, China, Indonesia, Myanmar, Nepal, Sri Lanka, Thailand and Vietnam.
Samuel S. Gnanamanickam is an Indian plant pathologist. He is known for his research on diversity of rice pathogens, molecular breeding of indica rices for disease resistance and for developing superior strains of beneficial strains of rhizosphere bacteria for biological control of rice diseases. He is a fellow of the National Academy of Agricultural Sciences and National Academy of Biological Sciences of India and was Chair of the biological control committee at the American Phytopathological Society. He was named by Marquis Who's Who as a noteworthy plant pathologist.
Puccinia coronata f. sp. avenae is the variation of the crown rust fungus which infects oat plants. Almost every growing region of oat has been affected by this pathogen at one point or another. During particularly bad epidemics, the worldwide crop yields have been reduced by up to 40%. One reason why Pca has such a prominent effect is that the conditions which favor oat production also favor the growth and inoculation of the rusts: Meaning that years in which the highest yields of crops are expected are the same years in which losses are the highest as well. Pca urediniospores germinate the best at temperature between 10–30 °C (50–86 °F) with germ-tube growth optimized at 20 °C (68 °F).