Adenosine triphosphate (ATP) is an organic compound that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the "molecular unit of currency" of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate (ADP) or to adenosine monophosphate (AMP). Other processes regenerate ATP. The human body recycles its own body weight equivalent in ATP each day. It is also a precursor to DNA and RNA, and is used as a coenzyme.

In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function.

Adenosine diphosphate (ADP), also known as adenosine pyrophosphate (APP), is an important organic compound in metabolism and is essential to the flow of energy in living cells. ADP consists of three important structural components: a sugar backbone attached to adenine and two phosphate groups bonded to the 5 carbon atom of ribose. The diphosphate group of ADP is attached to the 5’ carbon of the sugar backbone, while the adenine attaches to the 1’ carbon.

Glucose 6-phosphate is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms. With the chemical formula H(O)CCH(OH)CH2OPO32-, this anion is a monophosphate ester of glyceraldehyde.
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis. It is the phosphate ester of dihydroxyacetone.

Inosinic acid or inosine monophosphate (IMP) is a nucleotide. Widely used as a flavor enhancer, it is typically obtained from chicken byproducts or other meat industry waste. Inosinic acid is important in metabolism. It is the ribonucleotide of hypoxanthine and the first nucleotide formed during the synthesis of purine nucleotides. It can also be formed by the deamination of adenosine monophosphate by AMP deaminase. It can be hydrolysed to inosine.

Apyrase is a calcium-activated plasma membrane-bound enzyme that catalyses the hydrolysis of ATP to yield AMP and inorganic phosphate. Two isoenzymes are found in commercial preparations from S. tuberosum. One with a higher ratio of substrate selectivity for ATP:ADP and another with no selectivity.

Gramicidin S or Gramicidin Soviet is an antibiotic that is effective against some gram-positive and gram-negative bacteria as well as some fungi.

Phosphoenolpyruvate is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system.
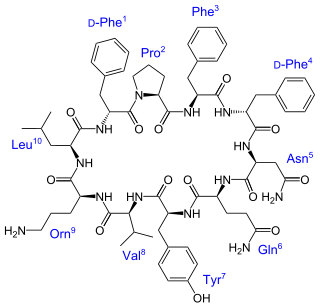
Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.

Fructose 1,6-bisphosphate, also known as Harden-Young ester, is fructose sugar phosphorylated on carbons 1 and 6. The β-D-form of this compound is common in cells. Upon entering the cell, most glucose and fructose is converted to fructose 1,6-bisphosphate.

The long chain fatty acyl-CoA ligase is an enzyme of the ligase family that activates the oxidation of complex fatty acids. Long chain fatty acyl-CoA synthetase catalyzes the formation of fatty acyl-CoA by a two-step process proceeding through an adenylated intermediate. The enzyme catalyzes the following reaction,
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.
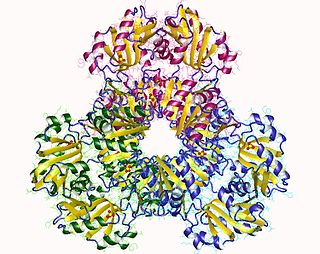
Ribose-phosphate diphosphokinase is an enzyme that converts ribose 5-phosphate into phosphoribosyl pyrophosphate (PRPP). It is classified under EC 2.7.6.1.
In enzymology, an alanine—tRNA ligase is an enzyme that catalyzes the chemical reaction

In enzymology, a phenylalanine—tRNA ligase is an enzyme that catalyzes the chemical reaction
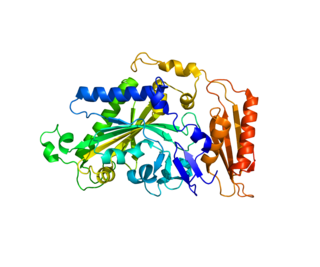
Phenylalanyl-tRNA synthetase, mitochondrial (FARS2) is an enzyme that in humans is encoded by the FARS2 gene. This protein encoded by FARS2 localizes to the mitochondrion and plays a role in mitochondrial protein translation. Mutations in this gene have been associated with combined oxidative phosphorylation deficiency 14, also known as Alpers encephalopathy, as well as spastic paraplegia 77 and infantile-onset epilepsy and cytochrome c oxidase deficiency.
Amino acid activation refers to the attachment of an amino acid to its respective transfer RNA (tRNA). The reaction occurs in the cell cytosol and consists of two steps: first, the enzyme aminoacyl tRNA synthetase catalyzes the binding of adenosine triphosphate (ATP) to a corresponding amino acid, forming a reactive aminoacyl adenylate intermediate and releasing inorganic pyrophosphate (PPi). Subsequently, aminoacyl tRNA synthetase binds the AMP-amino acid to a tRNA molecule, releasing AMP and attaching the amino acid to the tRNA. The resulting aminoacyl-tRNA is said to be charged.