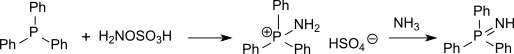
In chemistry a phosphine imide (sometimes abbreviated to phosphinimide) also known as a iminophosphorane is a functional group with the formula R3P=NR. While structurally related to phosphine oxide its chemistry has more in common with phosphonium ylides.
Contents
Anions of this group, with the structure R3P=N−, are called phosphinoimidates and are used as ligands to form phosphinimide complexes which are highly active catalysts in some olefin polymerization reactions. [1]