Related Research Articles
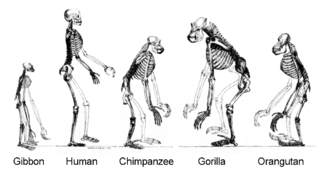
Human evolution is the evolutionary process within the history of primates that led to the emergence of Homo sapiens as a distinct species of the hominid family that includes all the great apes. This process involved the gradual development of traits such as human bipedalism, dexterity, and complex language, as well as interbreeding with other hominins, indicating that human evolution was not linear but weblike. The study of the origins of humans, variously known by the terms anthropogeny, anthropogenesis, or anthropogony, involves several scientific disciplines, including physical and evolutionary anthropology, paleontology, and genetics.

Homininae, also called "African hominids" or "African apes", is a subfamily of Hominidae. It includes two tribes, with their extant as well as extinct species: 1) the tribe Hominini ―and 2) the tribe Gorillini (gorillas). Alternatively, the genus Pan is sometimes considered to belong to its own third tribe, Panini. Homininae comprises all hominids that arose after orangutans split from the line of great apes. The Homininae cladogram has three main branches, which lead to gorillas and to humans and chimpanzees. There are two living species of Panina and two living species of gorillas, but only one extant human species. Traces of extinct Homo species, including Homo floresiensis have been found with dates as recent as 40,000 years ago. Organisms in this subfamily are described as hominine or hominines.

Homo habilis is an extinct species of archaic human from the Early Pleistocene of East and South Africa about 2.8 million years ago to 1.65 million years ago (mya). Upon species description in 1964, H. habilis was highly contested, with many researchers recommending it be synonymised with Australopithecus africanus, the only other early hominin known at the time, but H. habilis received more recognition as time went on and more relevant discoveries were made. By the 1980s, H. habilis was proposed to have been a human ancestor, directly evolving into Homo erectus which directly led to modern humans. This viewpoint is now debated. Several specimens with insecure species identification were assigned to H. habilis, leading to arguments for splitting, namely into "H. rudolfensis" and "H. gautengensis" of which only the former has received wide support.

The Paleolithic or Palaeolithic, also called the Old Stone Age, is a period in human prehistory that is distinguished by the original development of stone tools, and which represents almost the entire period of human prehistoric technology. It extends from the earliest known use of stone tools by hominins, c. 3.3 million years ago, to the end of the Pleistocene, c. 11,650 cal BP.

Homo ergaster is an extinct species or subspecies of archaic humans who lived in Africa in the Early Pleistocene. Whether H. ergaster constitutes a species of its own or should be subsumed into H. erectus is an ongoing and unresolved dispute within palaeoanthropology. Proponents of synonymisation typically designate H. ergaster as "African Homo erectus" or "Homo erectus ergaster". The name Homo ergaster roughly translates to "working man", a reference to the more advanced tools used by the species in comparison to those of their ancestors. The fossil range of H. ergaster mainly covers the period of 1.7 to 1.4 million years ago, though a broader time range is possible. Though fossils are known from across East and Southern Africa, most H. ergaster fossils have been found along the shores of Lake Turkana in Kenya. There are later African fossils, some younger than 1 million years ago, that indicate long-term anatomical continuity, though it is unclear if they can be formally regarded as H. ergaster specimens. As a chronospecies, H. ergaster may have persisted to as late as 600,000 years ago, when new lineages of Homo arose in Africa.

Homo rudolfensis is an extinct species of archaic human from the Early Pleistocene of East Africa about 2 million years ago (mya). Because H. rudolfensis coexisted with several other hominins, it is debated what specimens can be confidently assigned to this species beyond the lectotype skull KNM-ER 1470 and other partial skull aspects. No bodily remains are definitively assigned to H. rudolfensis. Consequently, both its generic classification and validity are debated without any wide consensus, with some recommending the species to actually belong to the genus Australopithecus as A. rudolfensis or Kenyanthropus as K. rudolfensis, or that it is synonymous with the contemporaneous and anatomically similar H. habilis.

Homo heidelbergensis is an extinct species or subspecies of archaic human which existed during the Middle Pleistocene. It was subsumed as a subspecies of H. erectus in 1950 as H. e. heidelbergensis, but towards the end of the century, it was more widely classified as its own species. It is debated whether or not to constrain H. heidelbergensis to only Europe or to also include African and Asian specimens, and this is further confounded by the type specimen being a jawbone, because jawbones feature few diagnostic traits and are generally missing among Middle Pleistocene specimens. Thus, it is debated if some of these specimens could be split off into their own species or a subspecies of H. erectus. Because the classification is so disputed, the Middle Pleistocene is often called the "muddle in the middle".

Homo is a genus of great ape that emerged from the genus Australopithecus and encompasses the extant species Homo sapiens and a number of extinct species classified as either ancestral or closely related to modern humans, including Homo erectus and Homo neanderthalensis. The oldest member of the genus is Homo habilis, with records of just over 2 million years ago. Homo, together with the genus Paranthropus, is probably most closely related to the species Australopithecus africanus within Australopithecus. The closest living relatives of Homo are of the genus Pan, with the ancestors of Pan and Homo estimated to have diverged around 5.7-11 million years ago during the Late Miocene.

Homo floresiensis( also known as "Flores Man") is an extinct species of small archaic human that inhabited the island of Flores, Indonesia, until the arrival of modern humans about 50,000 years ago.

The Lower Paleolithic is the earliest subdivision of the Paleolithic or Old Stone Age. It spans the time from around 3.3 million years ago when the first evidence for stone tool production and use by hominins appears in the current archaeological record, until around 300,000 years ago, spanning the Oldowan and Acheulean lithics industries.

The Steinheim skull is a fossilized skull of a Homo neanderthalensis or Homo heidelbergensis found on 24 July 1933 near Steinheim an der Murr, Germany.
Human taxonomy is the classification of the human species within zoological taxonomy. The systematic genus, Homo, is designed to include both anatomically modern humans and extinct varieties of archaic humans. Current humans have been designated as subspecies Homo sapiens sapiens, differentiated, according to some, from the direct ancestor, Homo sapiens idaltu.
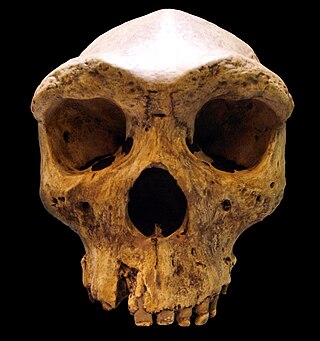
Archaic humans is a broad category denoting all species of the genus Homo that are not Homo sapiens. Among the earliest modern human remains are those from Jebel Irhoud in Morocco, Florisbad in South Africa (259 ka), and Omo-Kibish I in southern Ethiopia. Some examples of archaic humans include H. antecessor (1200–770 ka), H. bodoensis (1200–300 ka), H. heidelbergensis (600–200 ka), Neanderthals, H. rhodesiensis (300–125 ka) and Denisovans,

Post-canine megadontia is a relative enlargement of the molars and premolars compared to the size of the incisors and canines. This phenomenon is seen in some early hominid ancestors such as Paranthropus aethiopicus.

Homo erectus is an extinct species of archaic human from the Pleistocene, with its earliest occurrence about 2 million years ago. Its specimens are among the first recognizable members of the genus Homo.

Homo luzonensis, also locally called "Ubag" after a mythical caveman, is an extinct, possibly pygmy, species of archaic human from the Late Pleistocene of Luzon, the Philippines. Their remains, teeth and phalanges, are known only from Callao Cave in the northern part of the island dating to before 50,000 years ago. They were initially identified as belonging to modern humans in 2010, but in 2019, after the discovery of more specimens, they were placed into a new species based on the presence of a wide range of traits similar to modern humans as well as to Australopithecus and early Homo. In 2023, a study revealed that the fossilized remains of the Callao Man has been found out to be years old and much older than previously known.

Tautavel Man refers to the archaic humans which—from approximately 550,000 to 400,000 years ago—inhabited the Caune de l’Arago, a limestone cave in Tautavel, France. They are generally grouped as part of a long and highly variable lineage of transitional morphs which inhabited the Middle Pleistocene of Europe, and would eventually evolve into the Neanderthals. They have been variably assigned to either H. (s.?) heidelbergensis, or as a European subspecies of H. erectus as H. e. tautavelensis. The skull is reconstructed based on the specimens Arago 21 and 47, and it is, to a degree, more characteristic of what might be considered a typical H. erectus morphology than a typical H. heidelbergensis morphology. The brain capacity is 1,166 cc. They seem to have had an overall robust skeleton. Average height may have been 166 cm.

Several expansions of populations of archaic humans out of Africa and throughout Eurasia took place in the course of the Lower Paleolithic, and into the beginning Middle Paleolithic, between about 2.1 million and 0.2 million years ago (Ma). These expansions are collectively known as Out of Africa I, in contrast to the expansion of Homo sapiens (anatomically modern humans) into Eurasia, which may have begun shortly after 0.2 million years ago.

Homo naledi is an extinct species of archaic human discovered in 2013 in the Rising Star Cave system, Gauteng province, South Africa, dating to the Middle Pleistocene 335,000–236,000 years ago. The initial discovery comprises 1,550 specimens of bone, representing 737 different skeletal elements, and at least 15 different individuals. Despite this exceptionally high number of specimens, their classification with other Homo species remains unclear.

The archaeological site of Atapuerca is located in the province of Burgos in the north of Spain and is notable for its evidence of early human occupation. Bone fragments from around 800,000 years ago, found in its Gran Dolina cavern, provide the oldest known evidence of hominid settlement in Western Europe and of hominid cannibalism anywhere in the world.
References
- ↑ Ng, Andrew (4 June 2013). "Early Human Diets". California Academy of Sciences . Archived from the original on 13 April 2023. Retrieved 13 April 2023.
- ↑ Bogdan, Dennis (21 May 2020). "Comment - The End Of Meat Is Here - If you care about the working poor, about racial justice, and about climate change, you have to stop eating animals. - Jonathan Safran Foer". The New York Times . Archived from the original on 3 March 2021. Retrieved 13 April 2023.
- 1 2 3 4 5 Gowlett, J.A.J. (2003). "What Actually was the Stone Age Diet?". J. Nutr. Environ. Med. 13 (3): 143–147. doi:10.1080/13590840310001619338.
- ↑ Moorjani, Priya; Amorim, Carlos Eduardo G.; Arndt, Peter F.; Przeworski, Molly (2016). "Variation in the molecular clock of primates". Proceedings of the National Academy of Sciences. 113 (38): 10607–10612. Bibcode:2016PNAS..11310607M. doi: 10.1073/pnas.1600374113 . ISSN 0027-8424. PMC 5035889 . PMID 27601674.
- 1 2 3 Towle, Ian; Irish, Joel D.; Groote, Isabelle De (2017). "Behavioral inferences from the high levels of dental chipping in Homo naledi" (PDF). American Journal of Physical Anthropology. 164 (1): 184–192. doi:10.1002/ajpa.23250. ISSN 1096-8644. PMID 28542710. S2CID 24296825.
- 1 2 Marcé-Nogué, Jordi; Püschel, Thomas A.; Daasch, Alexander; Kaiser, Thomas M. (2020-04-22). "Broad-scale morpho-functional traits of the mandible suggest no hard food adaptation in the hominin lineage". Scientific Reports. 10 (1): 6793. Bibcode:2020NatSR..10.6793M. doi:10.1038/s41598-020-63739-5. ISSN 2045-2322. PMC 7176708 . PMID 32322020.
- ↑ Klein, R.G. (2013). "Stable carbon isotopes and human evolution". PNAS. 110 (26): 10470–2. Bibcode:2013PNAS..11010470K. doi: 10.1073/pnas.1307308110 . PMC 3696760 . PMID 23744041.
- ↑ Berthaume, Michael A.; Delezene, Lucas K.; Kupczik, Kornelius (2018-05-01). "Dental topography and the diet of Homo naledi" (PDF). Journal of Human Evolution. 118: 14–26. doi:10.1016/j.jhevol.2018.02.006. ISSN 0047-2484. PMID 29606200.
- ↑ Ungar, Peter S.; Berger, Lee R. (2018). "Brief communication: Dental microwear and diet of Homo naledi". American Journal of Physical Anthropology. 166 (1): 228–235. doi:10.1002/ajpa.23418. ISSN 1096-8644. PMID 29399788.
- ↑ Semaw, S.; Rogers, M.J.; Quade, J.; Renne, P.R.; Butler, R.F.; Dominguez-Rodrigo, M.; Stout, D.; Hart, W.S.; Pickering, T.; Simpson, S.W. (2003). "2.6-Million-year-old stone tools and associated bones from OGS-6 and OGS-7, Gona, Afar, Ethiopia". J. Hum. Evol. 45 (2): 169–177. doi:10.1016/S0047-2484(03)00093-9. PMID 14529651.
- 1 2 3 Ungar, P.S.; Grine, F.E.; Teaford, M.F.; El Zaatari, S. (2006). "Dental microwear and diets of African early Homo". J. Hum. Evol. 50 (1): 78–95. doi:10.1016/j.jhevol.2005.08.007. PMID 16226788.
- 1 2 Ungar, P.S.; Krueger, K.L.; Blumenschine, R.J.; Njau, J.; Scott, R.S. (2012). "Dental microwear texture analysis of hominins recovered by the Olduvai Landscape Paleoanthropology Project, 1995-2007". J. Hum. Evol. 63 (2): 429–437. doi:10.1016/j.jhevol.2011.04.006. PMID 21784504.
- ↑ Wood, B.; Strait, D. (2004). "Patterns of resource use in early Homo and Paranthropus". J. Hum. Evol. 46 (2): 119–162. doi:10.1016/j.jhevol.2003.11.004. PMID 14871560.
- ↑ Carotenuto, F.; Tsikaridze, N.; Rook, L.; Lordkipanidze, D.; Longo, L.; Condemi, S.; Raia, P. (2016). "Venturing out safely: The biogeography of Homo erectus dispersal out of Africa". J. Hum. Evol. 95: 1–12. doi:10.1016/j.jhevol.2016.02.005. hdl: 10356/82274 . PMID 27260171.
- 1 2 Perry, G.H.; Kistler, L.; Kelaita, M.A.; Sams, A.J. (2015). "Insights into hominin phenotypic and dietary evolution from ancient DNA sequence data". J. Hum. Evol. 79: 55–63. doi:10.1016/j.jhevol.2014.10.018. PMID 25563409.
- ↑ H. erectus cut, chewed way through evolution - Science News
- ↑ Brown, P.; Maeda, T. (2009). "Liang Bua Homo floresiensis mandibles and mandibular teeth: a contribution to the comparative morphology of a new hominin species". J. Hum. Evol. 57 (5): 571–596. doi:10.1016/j.jhevol.2009.06.002. PMID 19589559.
- ↑ Hillson, S.W.; Par, S.A.; Bello, S.M.; Roberts, M.B.; Stringer, C.B. (2010). "Two hominin incisor teeth from the middle Pleistocene site of Boxgrove, Sussex, England". Journal of Human Evolution. 59 (5): 493–503. doi:10.1016/j.jhevol.2010.06.004. PMID 20828787.
- ↑ Kupczik, K.; Hublin, J. (2010). "Mandibular molar root morphology in Neanderthals and Late Pleistocene and recent Homo sapiens". J. Hum. Evol. 59 (5): 525–541. doi:10.1016/j.jhevol.2010.05.009. PMID 20719359.
- 1 2 Perez-Perez, A.; de Castro, J.M.B.; de Lumley, M.A.; Turbon, D. (2003). "Non-occlusal dental microwear variability in a sample of Middle and Late Pleistocene human populations from Europe and the Near East". Journal of Human Evolution. 44 (4): 497–513. doi:10.1016/S0047-2484(03)00030-7. PMID 12727465.
- 1 2 Henry, A.G.; Brooks, A.S.; Piperno, D.R. (2011). "Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium)". Proc. Natl. Acad. Sci. U.S.A. 108 (2): 486–491. Bibcode:2011PNAS..108..486H. doi: 10.1073/pnas.1016868108 . PMC 3021051 . PMID 21187393.
- ↑ Sistiaga, A.; Mallol, C.; Galvin, B.; Summons, R.E. (2014). "The Neanderthal Meal : A New Perspective Using Faecal Biomarkers". PLOS ONE. 9 (6): 6–11. Bibcode:2014PLoSO...9j1045S. doi: 10.1371/journal.pone.0101045 . PMC 4071062 . PMID 24963925.
- ↑ Jaouen, Klervia; et al. (19 February 2019). "Exceptionally high δ15N values in collagen single amino acids confirm Neandertals as high-trophic level carnivores". Proceedings of the National Academy of Sciences of the United States of America . 116 (11): 4928–4933. Bibcode:2019PNAS..116.4928J. doi: 10.1073/pnas.1814087116 . PMC 6421459 . PMID 30782806.
- ↑ Yika, Bob (19 February 2019). "Isotopes found in bones suggest Neanderthals were fresh meat eaters". Phys.org . Retrieved 19 February 2019.
- ↑ Max Planck Institute for Evolutionary Anthropology (19 February 2019). "Neanderthals' main food source was definitely meat - Isotope analyses performed on single amino acids in Neanderthals' collagen samples shed new light on their debated diet". Science Daily . Retrieved 21 February 2019.
- 1 2 Gowlett, J.A.J.; Wrangham, R.W. (2013). "Earliest fire in Africa: towards the convergence of archaeological evidence and the cooking hypothesis". Azania Archaeol. Res. Africa. 48: 5–30. doi:10.1080/0067270X.2012.756754. S2CID 163033909.
- ↑ Hardy, K.; Brand-Miller, J.; Brown, K.D.; Thomas, M.G.; Copeland, L.; Dykhuizen, H.E.D.E. (2015). "The Importance of Dietary Carbohydrate in Human Evolution". Q. Rev. Biol. 90 (3): 251–268. doi:10.1086/682587. PMID 26591850. S2CID 28309169.
- ↑ Greaves, R.D.; Kramer, K.L. (2014). "Hunter-Gatherer Use Of Wild Plants And Domesticates: Archaeological Implications For Mixed Economies Before Agricultural Intensification". J. Archaeol. Sci. 41: 263–271. Bibcode:2014JArSc..41..263G. doi:10.1016/j.jas.2013.08.014.
- ↑ Will, M.; Parkington, J.E.; Kandel, A.W.; Conard, N.J. (2013). "Coastal adaptations and the Middle Stone Age lithic assemblages from Hoedjiespunt 1 in the Western Cape, South Africa". J. Hum. Evol. 64 (6): 518–537. doi:10.1016/j.jhevol.2013.02.012. PMID 23628196.
- ↑ Bocquet-Appel, J.P.; Demars, P.Y.; Noiret, L.; Dobrowsky, D. (2005). "Estimates of Upper Palaeolithic meta-population size in Europe from archaeological data". J. Archaeol. Sci. 32 (11): 1656–1668. Bibcode:2005JArSc..32.1656B. doi:10.1016/j.jas.2005.05.006.
- ↑ Cordain, L.; Eaton, S.; Miller, J.B.; Mann, N.; Hill, K. (2002). "The paradoxical nature of hunter-gatherer diets: meat-based, yet non-atherogenic". European Journal of Clinical Nutrition. 56: 42–52. doi: 10.1038/sj.ejcn.1601353 . PMID 11965522.
- ↑ Mayer, D.E.B.; Vandermeersch, B.; Bar-yosef, O. (2009). "Shells and ochre in Middle Paleolithic Qafzeh Cave, Israel : indications for modern behavior". J. Hum. Evol. 56 (3): 307–314. doi:10.1016/j.jhevol.2008.10.005. PMID 19285591.
- ↑ Kaplan, H.; Hill, K.I.M.; Lancaster, J.; Hurtado, A.M. (2000). "A Theory of Human Life History Evolution : Diet, Intelligence, and Longevity". Evol. Anthropol. 9 (4): 156–185. doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.3.CO;2-Z.
- ↑ Cole 2017, p. 1.
- ↑ Cole 2017, pp. 2–3.
- 1 2 Cole 2017, p. 3.
- ↑ Siefkes 2022, p. 133.
- ↑ Cole 2017, pp. 5, 7.
- ↑ Cole 2017, pp. 6–7.
- ↑ Nishimura, K.; Isoda, Y. (2004). "Evolution of cannibalism : referring to costs of cannibalism". Journal of Theoretical Biology. 226 (3): 291–300. Bibcode:2004JThBi.226..293N. doi:10.1016/j.jtbi.2003.09.007. PMID 14643643.
- ↑ Pare, Sascha (June 29, 2023). "Ancient human ancestors may have been cannibals, study on 1.45 million-year-old shinbone finds". livescience.com. Retrieved February 28, 2024.
- ↑ Saladié, Palmira; Huguet, Rosa; Rodríguez-Hidalgo, Antonio; Cáceres, Isabel; Esteban-Nadal, Montserrat; Arsuaga, Juan Luis; Bermúdez de Castro, José María; Carbonell, Eudald (November 1, 2012). "Intergroup cannibalism in the European Early Pleistocene: The range expansion and imbalance of power hypotheses". Journal of Human Evolution. 63 (5): 682–695. doi:10.1016/j.jhevol.2012.07.004. ISSN 0047-2484.
- ↑ Specktor, Brandon (April 3, 2020). "World's oldest human DNA found in 800,000-year-old tooth of a cannibal". livescience.com. Retrieved February 28, 2024.
- ↑ Hollingham, Richard (July 10, 2004). "Natural born cannibals". New Scientist. Retrieved February 28, 2024.
- ↑ Davis, Josh (October 4, 2023). "Oldest evidence of human cannibalism as a funerary practice". www.nhm.ac.uk. Retrieved February 28, 2024.
- ↑ Amos, Jonathan (February 16, 2011). "Ancient Britons 'drank from skulls'". BBC News. Retrieved February 28, 2024.
- ↑ Rougier, H.; Crevecoeur, I.; Beauval, C.; Posth, C.; Flas, D. (2016). "Neandertal cannibalism and Neandertal bones used as tools in Northern Europe". Scientific Reports. 6: 1–11. Bibcode:2016NatSR...629005R. doi:10.1038/srep29005. PMC 4933918 . PMID 27381450.
- ↑ Geggel, Laura (March 24, 2017). "Nom Nom Nom: Prehistoric Human Bones Show Signs of Cannibalism". livescience.com. Retrieved February 28, 2024.
- ↑ Rubinstein, William D. (November 18, 2020). "Life and Death in Pre-Contact Aboriginal Australia". quadrant.org.au. Retrieved March 24, 2024.
- ↑ Rubinstein, William D. (September 25, 2021). "The Incidence of Cannibalism in Aboriginal Society". quadrant.org.au. Retrieved March 24, 2024.
- ↑ Mathieson, I.; et al. (2015). "Genome-wide patterns of selection in 230 ancient Eurasians". Nature. 528 (7583): 499–503. Bibcode:2015Natur.528..499M. doi:10.1038/nature16152. PMC 4918750 . PMID 26595274.