Diterpenes are a class of terpenes composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and anti-inflammatory.
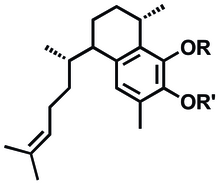
Forskolin (coleonol) is a labdane diterpene produced by the plant Coleus barbatus. Other names include pashanabhedi, Indian coleus, makandi, HL-362, mao hou qiao rui hua. As with other members of the large diterpene class of plant metabolites, forskolin is derived from geranylgeranyl pyrophosphate (GGPP). Forskolin contains some unique functional elements, including the presence of a tetrahydropyran-derived heterocyclic ring. Forskolin is commonly used in laboratory research to increase levels of cyclic AMP by stimulation of adenylate cyclase.
Aromatization is a chemical reaction in which an aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene. Aromatization includes the formation of heterocyclic systems.

Prodigiosin is the red dyestuff produced by many strains of the bacterium Serratia marcescens, as well as other Gram-negative, gamma proteobacteria such as Vibrio psychroerythrus and Hahella chejuensis. It is responsible for the pink tint occasionally found in grime that accumulates on porcelain surfaces such as bathtubs, sinks, and toilet bowls. It is in the prodiginine family of compounds which are produced in some Gram-negative gamma proteobacteria, as well as select Gram-positive Actinobacteria. The name prodigiosin is derived from prodigious.

Gliotoxin is a sulfur-containing mycotoxin that belongs to a class of naturally occurring 2,5-diketopiperazines produced by several species of fungi, especially those of marine origin. It is the most prominent member of the epipolythiopiperazines, a large class of natural products featuring a diketopiperazine with di- or polysulfide linkage. These highly bioactive compounds have been the subject of numerous studies aimed at new therapeutics. Gliotoxin was originally isolated from Gliocladium fimbriatum, and was named accordingly. It is an epipolythiodioxopiperazine metabolite that is one of the most abundantly produced metabolites in human invasive Aspergillosis (IA).

Steviol glycosides are the chemical compounds responsible for the sweet taste of the leaves of the South American plant Stevia rebaudiana (Asteraceae) and the main ingredients of many sweeteners marketed under the generic name stevia and several trade names. They also occur in the related species S. phlebophylla and in the plant Rubus chingii (Rosaceae).

Lanosterol synthase (EC 5.4.99.7) is an oxidosqualene cyclase (OSC) enzyme that converts (S)-2,3-oxidosqualene to a protosterol cation and finally to lanosterol. Lanosterol is a key four-ringed intermediate in cholesterol biosynthesis. In humans, lanosterol synthase is encoded by the LSS gene.

(S)-3,5-Dihydroxyphenylglycine or DHPG is a potent agonist of group I metabotropic glutamate receptors (mGluRs) mGluR1 and mGluR5.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.

Damascenones are a series of closely related chemical compounds that are components of a variety of essential oils. The damascenones belong to a family of chemicals known as rose ketones, which also includes damascones and ionones. beta-Damascenone is a major contributor to the aroma of roses, despite its very low concentration, and is an important fragrance chemical used in perfumery.

Phytoene is a 40-carbon intermediate in the biosynthesis of carotenoids. The synthesis of phytoene is the first committed step in the synthesis of carotenoids in plants. Phytoene is produced from two molecules of geranylgeranyl pyrophosphate (GGPP) by the action of the enzyme phytoene synthase. The two GGPP molecules are condensed together followed by removal of diphosphate and proton shift leading to the formation of phytoene.
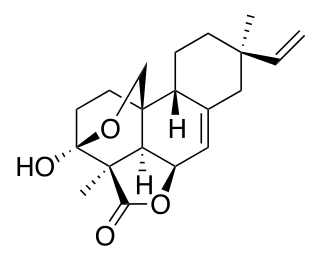
Momilactone B is an allelopathic agent produced from the roots of rice. It has been shown to be produced in high concentrations by the roots of rice seedlings. The production of momilactone B has also been induced in response to infection by blast fungus or irradiated with UV light. More recently it has been shown to be a potential chemotherapeutic agent against human colon cancer.

Macrophomic acid is a fungal metabolite isolated from the fungus Macrophoma commelinae. The enzyme macrophomate synthase converts 5-acetyl-4-methoxy-6-methyl-2-pyrone to 4-acetyl-3-methoxy-5-methyl-benzoic acid through an unusual intermolecular Diels-Alder reaction. The pathway to formation of macrophomic acid suggests that the enzyme is a natural Diels-Alderase. Formation of this type of aromatic ring compound normally proceeds via the shikimate and polyketide pathways; however, the production of macrophomic acid by macrophomate synthase proceeds totally differently. Learning about the production of macrophomic acid by a possible natural Diels-Alderase enzyme is important in understanding enzyme catalytic mechanisms. This knowledge can then be applied to organic synthesis.

Absinthin is a naturally produced triterpene lactone from the plant Artemisia absinthium (Wormwood). It constitutes one of the most bitter chemical agents responsible for absinthe's distinct taste. The compound shows biological activity and has shown promise as an anti-inflammatory agent, and should not be confused with thujone, a neurotoxin also found in Artemisia absinthium.

Pleuromutilin and its derivatives are antibacterial drugs that inhibit protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes.
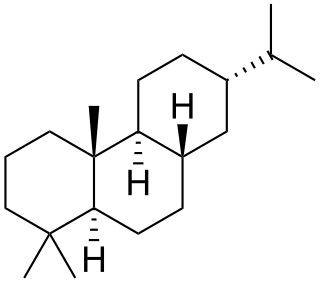
Abietane is a diterpene that forms the structural basis for a variety of natural chemical compounds such as abietic acid, carnosic acid, and ferruginol which are collectively known as abietanes or abietane diterpenes.

Nosiheptide is a thiopeptide antibiotic produced by the bacterium Streptomyces actuosus.

Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.

4-Hydroxyphenylglycine (HPG) is a non-proteogenic amino acid found in vancomycin and related glycopeptides. HPG is synthesized from the shikimic acid pathway and requires four enzymes to synthesize: Both L- and D-HPG are used in the vancomycin class of antibiotics. Tyrosine, a similar amino acid, differs by a methylene group (CH2) between the aromatic ring and the alpha carbon.