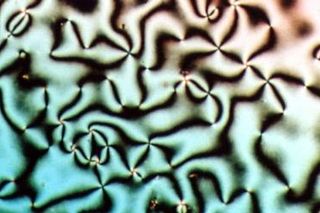
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in solid. There are many types of LC phases, which can be distinguished by their optical properties. The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter.

A polymer (;) is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals.

Soft matter or soft condensed matter is a subfield of condensed matter comprising a variety of physical systems that are deformed or structurally altered by thermal or mechanical stress of the magnitude of thermal fluctuations. These materials share an important common feature in that predominant physical behaviors occur at an energy scale comparable with room temperature thermal energy, and that entropy is considered the dominant factor. At these temperatures, quantum aspects are generally unimportant. Soft materials include liquids, colloids, polymers, foams, gels, granular materials, liquid crystals, flesh, and a number of biomaterials. When soft materials interact favorably with surfaces, they become squashed without an external compressive force. Pierre-Gilles de Gennes, who has been called the "founding father of soft matter," received the Nobel Prize in Physics in 1991 for discovering that methods developed for studying order phenomena in simple systems can be generalized to the more complex cases found in soft matter, in particular, to the behaviors of liquid crystals and polymers.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation.
In chemistry, photoisomerization is a form of isomerization induced by photoexcitation. Both reversible and irreversible photoisomerizations are known for photoswitchable compounds. The term "photoisomerization" usually, however, refers to a reversible process.

In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another.

Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra.

Pentacene is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet (UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light.
A photoswitch is a type of molecule that can change its structural geometry and chemical properties upon irradiation with electromagnetic radiation. Although often used interchangeably with the term molecular machine, a switch does not perform work upon a change in its shape whereas a machine does. However, photochromic compounds are the necessary building blocks for light driven molecular motors and machines. Upon irradiation with light, photoisomerization about double bonds in the molecule can lead to changes in the cis- or trans- configuration. These photochromic molecules are being considered for a range of applications.
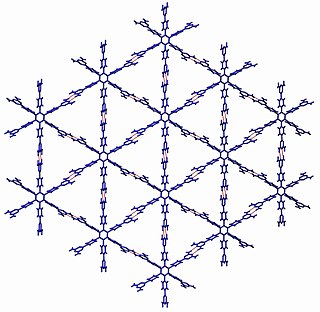
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of intermolecular interactions. It is an interdisciplinary academic field, bridging solid-state and supramolecular chemistry.
In nuclear chemistry and nuclear physics, J-couplings are mediated through chemical bonds connecting two spins. It is an indirect interaction between two nuclear spins that arises from hyperfine interactions between the nuclei and local electrons. In NMR spectroscopy, J-coupling contains information about relative bond distances and angles. Most importantly, J-coupling provides information on the connectivity of chemical bonds. It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.
In polymer chemistry and materials science, the term "polymer" refers to large molecules whose structure is composed of multiple repeating units. Supramolecular polymers are a new category of polymers that can potentially be used for material applications beyond the limits of conventional polymers. By definition, supramolecular polymers are polymeric arrays of monomeric units that are connected by reversible and highly directional secondary interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions, π-π stacking, metal coordination, halogen bonding, chalcogen bonding, and host–guest interaction. The direction and strength of the interactions are precisely tuned so that the array of molecules behaves as a polymer in dilute and concentrated solution, as well as in the bulk.
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines.

Lyotropic liquid crystals result when fat-loving and water-loving chemical compounds known as amphiphiles dissolve into a solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water.

Ferroelectric polymers are a group of crystalline polar polymers that are also ferroelectric, meaning that they maintain a permanent electric polarization that can be reversed, or switched, in an external electric field.
A liquid-crystal laser is a laser that uses a liquid crystal as the resonator cavity, allowing selection of emission wavelength and polarization from the active laser medium. The lasing medium is usually a dye doped into the liquid crystal. Liquid-crystal lasers are comparable in size to diode lasers, but provide the continuous wide spectrum tunability of dye lasers while maintaining a large coherence area. The tuning range is typically several tens of nanometers. Self-organization at micrometer scales reduces manufacturing complexity compared to using layered photonic metamaterials. Operation may be either in continuous wave mode or in pulsed mode.
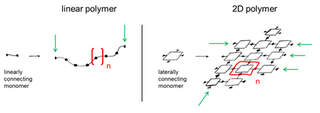
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.
A spiropyran is a type of organic chemical compound, known for photochromic properties that provide this molecule with the ability of being used in medical and technological areas. Spiropyrans were discovered in the early twentieth century. However, it was in the middle twenties when Fisher and Hirshbergin observed their photochromic characteristics and reversible reaction. In 1952, Fisher and co-workers announced for the first time photochromism in spiropyrans. Since then, there have been many studies on photochromic compounds that have continued up to the present.
Polymerization-induced phase separation (PIPS) is the occurrence of phase separation in a multicomponent mixture induced by the polymerization of one or more components. The increase in molecular weight of the reactive component renders one or more components to be mutually immiscible in one another, resulting in spontaneous phase segregation.

β-Butyrolactone is the intramolecular carboxylic acid ester (lactone) of the optically active 3-hydroxybutanoic acid. It is produced during chemical synthesis as a racemate. β-Butyrolactone is suitable as a monomer for the production of the biodegradable polyhydroxyalkanoate poly(3-hydroxybutyrate) (PHB). Polymerisation of racemic (RS)-β-butyrolactone provides (RS)-polyhydroxybutyric acid, which, however, is inferior in essential properties (e.g. strength or degradation behaviour) to the (R)-poly-3-hydroxybutyrate originating from natural sources.












