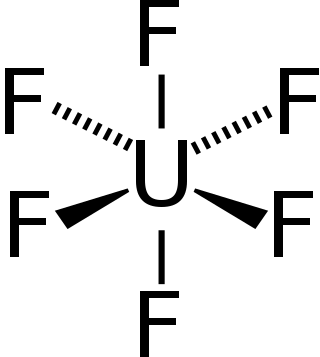
Uranium hexafluoride, sometimes called hex, is an inorganic compound with the formula UF6. Uranium hexafluoride is a volatile white solid that reacts with water, releasing corrosive hydrofluoric acid. The compound reacts mildly with aluminium, forming a thin surface layer of AlF3 that resists any further reaction from the compound. UF6 is used in the process of enriching uranium, which produces fuel for nuclear reactors and nuclear weapons.
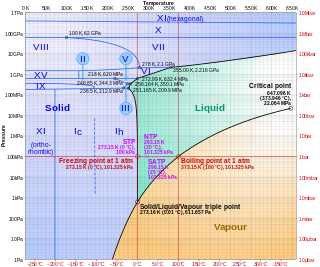
Ice Ic is a metastable cubic crystalline variant of ice. Hans König was the first to identify and deduce the structure of ice Ic. The oxygen atoms in ice Ic are arranged in a diamond structure and is extremely similar to ice Ih having nearly identical densities and the same lattice constant along the hexagonal puckered-planes. It forms at temperatures between 130 and 220 kelvins upon cooling, and can exist up to 240 K (−33 °C) upon warming, when it transforms into ice Ih.
The Dennis Gabor Medal and Prize is a prize awarded biannually by the Institute of Physics for distinguished contributions to the application of physics in an industrial, commercial or business context. The medal is made of silver and is accompanied by a prize and a certificate.

Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable over a wide range of temperatures and pressures and transforms into low-density amorphous ice (LDA) above 120 K (−153 °C). Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least 715 K (442 °C) and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt.
John Stephen Loveday is an experimental physicist working in high pressure research. He was educated at Coopers School in Chislehurst and at the University of Bristol, from where he took his PhD in Physics. He currently works as a Reader in the School of Physics and Astronomy at the University of Edinburgh, Scotland.
William (Bill) Cochran was a Scottish physicist. He is best known for "pioneering contributions to the science of X-ray crystallography", for which he was awarded the Hughes Medal in 1978.
Colin Edward Webb is a British physicist and former professor at the University of Oxford, specialising in lasers.

Boron can be prepared in several crystalline and amorphous forms. Well known crystalline forms are α-rhombohedral (α-R), β-rhombohedral (β-R), and β-tetragonal (β-T). In special circumstances, boron can also be synthesized in the form of its α-tetragonal (α-T) and γ-orthorhombic (γ) allotropes. Two amorphous forms, one a finely divided powder and the other a glassy solid, are also known. Although at least 14 more allotropes have been reported, these other forms are based on tenuous evidence or have not been experimentally confirmed, or are thought to represent mixed allotropes, or boron frameworks stabilized by impurities. Whereas the β-rhombohedral phase is the most stable and the others are metastable, the transformation rate is negligible at room temperature, and thus all five phases can exist at ambient conditions. Amorphous powder boron and polycrystalline β-rhombohedral boron are the most common forms. The latter allotrope is a very hard grey material, about ten percent lighter than aluminium and with a melting point (2080 °C) several hundred degrees higher than that of steel.

Prof John Millar Thomson PIC FRS FRSE LLD was a British chemist who held various leading positions with British chemical societies and was the vice-principal of King's College London. He was President of the Institute of Chemistry from 1900 to 1903.

Robert Joseph Cava is a solid-state chemist at Princeton University where he holds the title Russell Wellman Moore Professor of Chemistry. Previously, Professor Cava worked as a staff scientist at Bell labs from 1979–1996, where earned the title of Distinguished Member of the Technical Staff. As of 2016 his research investigates topological insulators, semimetals, superconductors, frustrated magnets and thermoelectrics.

Polly Louise Arnold is director of the chemical sciences division at Lawrence Berkeley National Laboratory and professor of chemistry at the University of California, Berkeley. She previously held the Crum Brown chair in the School of Chemistry, University of Edinburgh from 2007 to 2019 and an Engineering and Physical Sciences Research Council (EPSRC) career fellowship.

John Paul Attfield is a Professor of Materials science in the School of Chemistry at the University of Edinburgh and Director of the Centre for Science at Extreme Conditions (CSEC).

R. J. Dwayne Miller is a Canadian chemist and a professor at the University of Toronto. His focus is in physical chemistry and biophysics. He is most widely known for his work in ultrafast laser science, time-resolved spectroscopy, and the development of new femtosecond electron sources. His research has enabled real-time observation of atomic motions in materials during chemical processes and has shed light on the structure-function correlation that underlies biology.
Andrew Dawson Taylor was director of the Science and Technology Facilities Council National Laboratories – Rutherford Appleton Laboratory, Daresbury Laboratory, and the UK Astronomy Technology Centre in Edinburgh until his retirement in 2019.

Andrew Harrison OBE is a British chemist and a research manager. From 1978 he studied chemistry at Oxford University, where he graduated as PhD in 1985. Then he worked as a researcher in Britain, Canada and France. In October 2020 he became an Officer of the Order of the British Empire.

Ross John Angel is an internationally recognized researcher in mineralogy, expert in crystallography and elastic properties of geological materials and key industrial materials, which he studies with experimental and analytical approaches. He is the lead author or co-author of over 240 articles in international scientific journals, he received the Dana Medal from the Mineralogical Society of America in 2011 and is currently a director of research at the Institute of Geosciences and Geo-resources of the National Research Council (Italy).

Paul Francis McMillan was a British chemist who held the Sir William Ramsay Chair of Chemistry at University College London. His research considered the study of matter under extreme conditions of temperature and pressure, with a focus on phase transitions, amorphisation, and the study of glassy states. He has also investigated the survival of bacteria and larger organisms (tardigrades) under extreme compression, studies of amyloid fibrils, the synthesis and characterisation of carbonitride nanocrystals and the study of water motion in confined environments. He has made extensive use of Raman spectroscopy together with X-ray diffraction and neutron scattering techniques.
Ice XIX is a proposed crystalline phase of water. Along with ice XV, it is one of two phases of ice directly related to ice VI. Ice XIX is prepared by cooling HCl-doped ice VI at a pressure above 1.6 GPa down to about 100 K. As of 2022, its crystal structure has not been elucidated.
Ice IV is a metastable high-pressure phase of ice. It is formed when liquid water is compressed with an immense force.

Thomas Loerting is an Austrian chemist and associate professor at the University of Innsbruck. His research focuses on amorphous systems, the physics and chemistry of ice and chemistry at low temperatures.












